A Conservation Action Plan for Bicknell's Thrush (Catharus bicknelli)
Editors
Plan contributors
| Yves Aubry | Canadian Wildlife Service, Environment and Climate Change Canada |
| Jorge Brocca | SOH Conservación |
| Greg Campbell | Bird Studies Canada |
| James E. Goetz | Cornell Lab of Ornithology |
| Jérôme Lemaître | Ministère des Forêts, de la Faune et des Parcs Québec |
| John D. Lloyd | Vermont Center for Ecostudies |
| Kent P. McFarland | Vermont Center for Ecostudies |
| Leighlan Prout | White Mountain National Forest |
| Juan C. Martínez-Sánchez | Vermont Center for Ecostudies |
| Jose Almonte | Ministerio de Medio Ambiente Y Recursos Naturales de la República Dominicana |
| Greg Butcher | U.S. Forest Service |
| Randy Dettmers | U.S. Fish and Wildlife Service |
| Charles Francis | Canadian Wildlife Service, Environment and Climate Change Canada |
| Dan Hudnutt | Wagner Forest Management |
| Eduardo E. Iñigo-Elias | Cornell Lab of Ornithology |
| Marie-France Julien | Regroupement QuébecOiseaux |
| Yolanda Leon | Grupo Jaragua |
| Anthony Tur | U.S. Fish and Wildlife Service |
| Chelsae Postma | University of New Brunswick |
| Geneviève Perreault | Regroupement QuébecOiseaux |
| Christopher C. Rimmer | Vermont Center for Ecostudies |
| Andrew Rothman | American Bird Conservancy |
| Peter Thomas | Canadian Wildlife Service, Environment and Climate Change Canada |
| Junior A. Tremblay | Science and Technology Branch, Environment and Climate Change Canada |
| Becky Whittam | Canadian Wildlife Service, Environment and Climate Change Canada |
Preface
 “A Conservation Action Plan for Bicknell’s Thrush (Catharus bicknelli)”, released in 2010, was the result of a broad collaboration among NGOs, government agencies, the forest-products industry, and academics who united under the banner of the International Bicknell’s Thrush Conservation Group, or IBTCG. The Plan set ambitious goals to increase the abundance and distribution of Bicknell’s Thrush and identified actions that would help achieve them. It was also written with full awareness that conservation plans can quickly become obsolete in the fast-shifting landscape of conservation. To ensure the continued relevance of the Plan, the IBTCG set a goal of reviewing and updating the Plan every five years, or sooner as warranted.
“A Conservation Action Plan for Bicknell’s Thrush (Catharus bicknelli)”, released in 2010, was the result of a broad collaboration among NGOs, government agencies, the forest-products industry, and academics who united under the banner of the International Bicknell’s Thrush Conservation Group, or IBTCG. The Plan set ambitious goals to increase the abundance and distribution of Bicknell’s Thrush and identified actions that would help achieve them. It was also written with full awareness that conservation plans can quickly become obsolete in the fast-shifting landscape of conservation. To ensure the continued relevance of the Plan, the IBTCG set a goal of reviewing and updating the Plan every five years, or sooner as warranted.
In November 2015, five years after the first Plan was released, the IBTCG met once again in Woodstock, Vermont—site of the inaugural IBTCG meeting in 2007—to begin the process of revising the Plan. The two-day workshop highlighted significant progress towards the goals of the Plan, but also reinforced the need for continued action on behalf of Bicknell’s Thrush and its habitat. Actions taken by the IBTCG since the release of the first Plan have mitigated some important threats to Bicknell’s Thrush, but others remain largely unabated. As such, the IBTCG recognized the need for an updated and revised Plan that would catalyze action and guide the collective efforts of those interested in conservation of Bicknell’s Thrush.
This revised Plan reflects the consensus of the IBTCG about the primary threats facing Bicknell’s Thrush and the actions that may help mitigate those threats. It does not provide a comprehensive list of every threat to Bicknell’s Thrush, but instead attempts to focus on those believed to pose the greatest risk of further endangerment of the species. It does not identify every action that might prove useful in mitigating threats; rather, it singles out actions believed to have a higher probability of success based on published research or the personal experience of contributors to the revised Plan. The revised Plan is a tool for communicating about the conservation of Bicknell’s Thrush, both within the community of scientists and conservation practitioners that make up IBTCG and more broadly to policy makers, elected officials, and the public. Finally, the revised Plan is intended as a guide to investing limited resources for conservation most effectively.
John D. Lloyd
Kent P. McFarland
Editors
Abbreviations
IBTCG……….International Bicknell’s Thrush Conservation Group
BMP………….Best Management Practice
BSC/EOC…..Bird Studies Canada/Études d’Oiseaux Canada
CWS………….Canadian Wildlife Service
ECCC…………Environment and Climate Change Canada
NP……………..National Park
VCE……………Vermont Center for Ecostudies
COSEWIC…..Committee on the Status of Endangered Wildlife in Canada
BBS……………Breeding Bird Survey
WMNF……….White Mountain National Forest
USFWS………US Fish and Wildlife Service
IUCN………….The International Union for Conservation of Nature and Natural Resources
NGO…………..Non-Governmental Organization
MBW………….Mountain Birdwatch
Suggested Citation
Lloyd, John D. and Kent P. McFarland, editors. 2017. A Conservation Action Plan for Bicknell’s Thrush (Catharus bicknelli). International Bicknell’s Thrush Conservation Group (IBTCG). http://bicknellsthrush.org/conservation-action-plan/conservation-action-plan-for-bicknells-thrush. 10.6084/m9.figshare.4962608
Executive Summary
The revised Conservation Action Plan for Bicknell’s Thrush (Catharus bicknelli) provides the updated consensus of the International Bicknell’s Thrush Conservation Group about the primary threats facing Bicknell’s Thrush and the actions that may help mitigate those threats. The first Conservation Action Plan was released in 2010 and had a planned lifespan of 5 years.
The goals set out in the first Plan have not changed: to increase population size by 25% by 2060 and to maintain or increase the extent of breeding occurrence.
The primary threats to Bicknell’s Thrush also remain largely the same. The three most significant threats to the viability of Bicknell’s Thrush are:
- The clearing and burning of forests in the Dominican Republic and Haiti to create agricultural fields.
- Incompatible forestry practices on the breeding grounds, especially in Canada.
- Climate change.
Agricultural expansion into forested areas on the wintering grounds and incompatible forestry practices on the breeding grounds both result in a direct loss of habitat. Over the long term, unmitigated climate change is expected to eliminate suitable habitat over most of the current breeding distribution. The effects of climate change on wintering habitat are less certain.
Deforestation on the wintering grounds occurs both within and outside of established protected areas. Priority actions to address loss of wintering habitat within protected areas include maintaining and strengthening enforcement of protected-area boundaries and training to increase institutional capacity for management of protected areas. Priority actions to address loss of wintering habitat more generally include protection of potential habitat through purchase or easement agreements, supporting compatible land uses via direct economic incentives, disincentivizing incompatible land uses by influencing market forces, and providing livelihood alternatives that are linked to intact forest ecosystems.
The impact of incompatible forestry practices on breeding habitat has been somewhat mitigated since the first Conservation Action Plan and conservation actions identified in the revised Plan seek to build on this initial success. In particular, we recommend expanded implementation of existing best-management practices for forestry operations that are conducted in habitat for Bicknell’s Thrush and, where necessary, expanded implementation of policies that require avoiding or limiting use of pre-commercial thinning in Bicknell’s Thrush habitat. Further research on how forest management can be used to create breeding habitat for Bicknell’s Thrush is also warranted.
Climate change is likely to affect Bicknell’s Thrush populations through a variety of direct and indirect pathways. The most significant threat posed by climate change is the expected disappearance of the balsam fir (Abies balsamea) forests in which Bicknell’s Thrush raise their offspring. Models predict that most or all breeding habitat for Bicknell’s Thrush is at risk from climate change due to the sensitivity of balsam fir to increased temperature. Hardwood forests, which are not habitable by Bicknell’s Thrush, are expected to expand in response to climate change and replace forests of balsam fir, resulting in significant loss of breeding habitat. Actions identified to address this threat were indirect, and included supporting policies that lead to a reduction in greenhouse-gas concentrations and conducting research on how to mitigate the effects of climate change.
In addition to actions designed to address these high-priority threats, we also discuss actions that can address other important threats, including development of wind-energy facilities, charcoal production on the wintering grounds, and the direct and indirect effects of invasive mammals in wintering habitat.
Chapter 1. Background
In 1939, the esteemed ornithologist George Wallace wrote, “The discouragingly dense tangles in which Bicknell’s thrushes dwell have kept their habits long wrapped in mystery” (Wallace 1939:285). Intensive research and monitoring over the last several decades by dedicated members of the International Bicknell’s Thrush Conservation Group (IBTCG) have solved many, but not all, of these mysteries.
Bicknell’s Thrush (Catharus bicknelli), classified as a subspecies of Gray-cheeked Thrush (C. minimus) following its 1881 discovery in New York’s Catskill Mountains, gained full species status in 1995 (Monroe et al. 1995). It is among North America’s rarest and most range-restricted breeding birds.
Detailed information on the life history characteristics of Bicknell’s Thrush is available in the recently updated Birds of North America Online species account (Townsend et al. 2015). Here, we summarize pertinent information on the species’ biology and ecology, highlighting new findings or those directly relevant to conservation.
Distribution
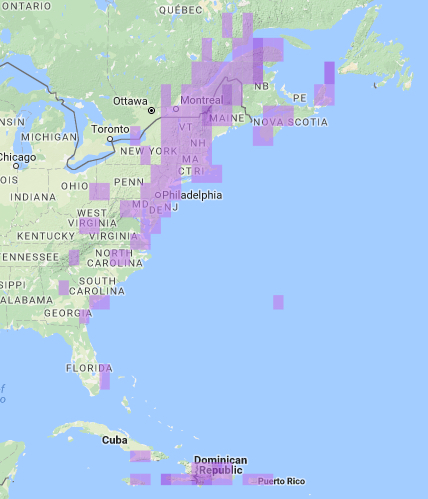
eBird data provides a detailed map of the range of this species (see eBird Year-round Range and Point Map for Bicknell’s Thrush).
Breeding Range
Bicknell’s Thrush occupies a restricted and highly fragmented breeding range. It is found in several mountains and highlands of southern Québec northwest of the St. Lawrence River, southeast of the river, and in the mountains in southern Québec along the border with the U.S. (e.g., Mont Gosford, NP du Mont-Mégantic) (Ouellet 1993); in northwest and north-central New Brunswick, and Cape Breton Island, Nova Scotia, including the small, outlying St. Paul Island, but only rarely along coastal New Brunswick and coastal Nova Scotia (Erskine 1992, Whittam 2015). In the U.S., it is found in the mountains of central and western Maine (Adamus 1987, Atwood et al. 1996), the White Mountains in New Hampshire (Richards and Foss 1994, Atwood et al. 1996), the Green Mountains and the northeast highlands in Vermont (Kibbe 1985, Atwood et al. 1996), and the Adirondack and Catskill Mountains, New York (Peterson 1988, Atwood et al. 1996).
Several local extirpations were documented during the twentieth century. An historic breeding population disappeared from Mt. Greylock, Massachusetts (10 pairs in 1950s to 0 in 1973) (Veit and Petersen 1993); on Seal and Mud islands, Nova Scotia (Wallace 1939, Erskine 1992); on Cape Forchu in southwest Nova Scotia; in Fundy National Park, New Brunswick (Christie 1993); and Grand Manan Island, New Brunswick. A population present on the Magdalen Islands, Québec during the first breeding bird atlas of Québec (Ouellet 1996) was not located during the second atlas (Quebec Breeding Bird Atlas, unpubl. data). Further range contraction in the Canadian Maritime Provinces occurred between 1990 and 2010, with the Second Atlas of Breeding Birds showing 50% fewer occupied sites than during the first Atlas (Whittam 2015). The continued presence of Bicknell’s Thrush, however, was confirmed on 63 of 73 historic (pre-1992) U.S. breeding sites surveyed in 1992–1995 (Atwood et al. 1996).
Winter Range
The known wintering distribution of Bicknell’s Thrush is confined to the Greater Antilles. Modeling of over-winter habitat selection and environmental factors indicated that 51% of appropriate and available wintering habitat occurs in Dominican Republic, 15.1% in Cuba, 13.5% in Jamaica, 10.6% in Haiti, and 9.9% in Puerto Rico (McFarland et al. 2013), although more recent surveys suggest that this may be an overestimate for Puerto Rico as very few birds appear to winter on the island (VCE, unpubl. data). Specimen and field-survey data indicate that the bulk of the wintering population occurs within the Dominican Republic (Wetmore and Swales 1931, Ouellet 1993, Rimmer et al. 1999) where Bicknell’s Thrush is widely distributed and locally common from sea level to 2,220 m (Rimmer et al. 1999, Townsend et al. 2015). Records from Haiti indicate the species is restricted to higher elevations, mainly in the southwest (Massif de la Hotte) and the east (Massif La Visite) (Wetmore and Swales 1931, Woods and Ottenwalder 1983, 1986; Rimmer and Goetz 2010). It is uncommon and local in Jamaica and likely persists mainly in the Blue Mountains from 1,200 to 2,225 m elevation, although recent, extensive surveys are lacking (Townsend et al. 2015). Recorded in eastern Cuba at 1,600–1,960 m in Sierra Maestra (Rompre et al. 1999, Rompré et al. 2000, Llanes Sosa et al. 2003). The two October specimens from Havana, Cuba in 1960s (Garrido and Garcia Montaña 1975) probably represent transients. Surveys and banding operations confirm that Bicknell’s Thrush is a rare and local winter resident on Puerto Rico. Scattered individuals have been found in the Luquillo Mountains at 450–720 m elevation and in Sierra de Cayey at 720 m (Arendt 1992). Two birds were captured in Guanica State Forest and Biosphere Reserve, one in 1985 and one in 2005, both in karstic, dry forest habitat (J. Faaborg, pers. comm.). Intensive surveys conducted in 2015 and 2016 in a variety of forested habitats at all elevations detected 11 Bicknell’s thrushes, all at high elevations in Cordillera Central, from Guilarte State Forest (~1,036 m elevation) and Cerro Morales (823 m) in the west to the Cerro Punta area (1,189-1,250 m) (VCE, unpubl. data).
Migration
Until recently, little information existed for identifying migration routes of Bicknell’s Thrush. Generally, individuals follow the eastern flyway along the coast of North America. Banding records and geolocator data suggest that Bicknell’s Thrush follow an over-water route on fall migration, leaving from coastal Virginia and North Carolina and heading directly to the Caribbean (Townsend et al. 2015). In contrast, spring banding, nocturnal flight call recordings, and geolocator data indicate that many individuals migrate westward to Florida and then northward along the coast, overland to the breeding grounds (Evans 1994, Townsend et al. 2015). Some individuals with geolocators did travel northward from the winter grounds to the central East Coast and then overland to the breeding grounds (Townsend et al. 2015).
Habitat
Breeding
Bicknell’s Thrush is a habitat specialist found primarily in montane forests dominated by balsam fir (Abies balsamea), with lesser amounts of spruce (Picea spp.), heart-leaved paper birch (Betula cordifolia), mountain ash (Sorbus spp.), and other hardwood species (Townsend et al. 2015). Bicknell’s Thrush generally breeds above 1,100 m elevation at the southern extent of its range in the Catskills Mountains of New York and as low as 380 m in several highlands in eastern Québec and Atlantic Canada (Bird Studies Canada/Études d’Oiseaux Canada [BSC/EOC], unpubl. data). In highlands of Québec, the lower elevational limit of its distribution is typically between 600 and 850 m (MDDEFP 2013).
Bicknell’s Thrush is often associated with disturbed areas undergoing vigorous succession, characterized by standing dead conifers and dense regrowth of balsam fir (Wallace 1939, Townsend et al. 2015). Highest densities are typically found in chronically disturbed stands of dense, stunted fir on exposed ridgelines or along edges of human-created openings (e.g., ski trails in the US), or in regenerating fir waves. Bicknell’s Thrush also occupies regenerating stands of mixed forest following forest fires or clear-cutting in industrial forest in the highlands of Québec, New Brunswick, and Nova Scotia (Ouellet 1993, Nixon et al. 2001, Connolly et al. 2002, Leonard and Chisholm 2008). Occupied patches in industrial forest have a high density of small-diameter (5-10 cm) trees (38,000 – 64,000 stems per ha) with a significant component of balsam fir (24% – 65%) and white birch (B. papyrifera) (22% – 45%) (Nixon et al. 2001, Chisholm and Leonard 2008, Aubry et al. 2011, McKinnon et al. 2014). The species cannot, therefore, be characterized as a forest-interior species, but appears instead to be adapted to the patch dynamics of mosaic forests where forest structure is heavily influenced by both natural disturbance and forestry activities (Aubry et al. 2011).
The species is also found in coastal maritime spruce-fir forests near sea level in New Brunswick, Nova Scotia (Erskine 1992), and Québec (Ouellet 1996) where cool sea breezes and higher precipitation levels maintain dense spruce-fir stands selected locally by Bicknell’s Thrush. However, results obtained during the Second Atlas of Breeding Birds of the Maritime Provinces (Whittam 2015), as well as in the second atlas from Québec (Québec Breeding Bird Atlas), suggest that Bicknell’s Thrush has been extirpated from many coastal sites once occupied by the species.
Winter
Bicknell’s Thrush primarily inhabits mesic to wet broadleaf montane forests in the Dominican Republic (Rimmer et al. 1999), Haiti (Rimmer et al. 2005, Rimmer and Goetz 2010), Cuba (Rompré et al. 2000), Jamaica (McFarland et al. 2013), and Puerto Rico (McFarland et al. 2013). In the Dominican Republic, the species is found from sea level to 2,200 m, although >60% of occupied sites were in forests >1,000 m elevation, likely due to habitat loss at lower elevations (Townsend et al. 2015). The majority (75%) of occupied sites were in broadleaf-dominated forests at all elevations, 19% were in mixed broadleaf-pine forests, and 6% occurred in pine-dominated forests. The use of regenerating secondary forests (22% of occupied sites) in the Dominican Republic indicates some degree of flexibility in habitat use in response to the widespread loss and degradation of preferred primary broadleaf forest habitat.
In the Dominican Republic, evidence exists for sexual habitat segregation (Townsend et al. 2009). In the Sierra de Bahoruco, in predominantly undisturbed broadleaf montane forests, males outnumber females by a 4:1 ratio, whereas a population at a mid-elevation, moderately disturbed wet forest site in the Cordillera Septentrional showed a 1:1 sex ratio. No significant differences exist between male and female mean territory size at either site, but females in the Cordillera Septentrional are in better physiological condition relative to females in the Sierra de Bahoruco (Townsend et al. 2011).
Migratory Stopover
There is little published information on habitat selection during migration, but migrants have been documented at an array of coastal and inland sites, suggesting little specificity in regards to habitat use other than that the area is forested (Townsend et al. 2015).
Life History and Demography
Mating System and Sex Ratio
The mating system of Bicknell’s Thrush is unusual and appears closest to “female-defense polygynandry”, in which both males and females mate with multiple partners, multiple paternity is common, and nestlings are most often fed by more than one male (Goetz et al. 2003). In Vermont, most broods (>75%) have mixed paternity and some males sire offspring in multiple nests during the same breeding season. This unusual mating system results in a lack of territoriality among males. Consequently, estimation of breeding densities by traditional methods is difficult, since males do not sing regularly to defend territories and may be present when no singing is heard.
Based on mist-net capture data, the mean sex ratio among breeding adults in Vermont over eight years was >2 males for every female (Townsend et al. 2009). However, the sex ratio of nestlings and fledglings was nearly 1:1, with a slight skew toward females. In two separate Québec breeding populations studied over four years (2002–2005), sex ratios of adults and nestlings were similar to those in Vermont (Y. Aubry, CWS, unpubl. data). The cause of a range-wide male-biased sex ratio is not known, but may relate to differential post-natal dispersal patterns and survivorship, survival of first-year birds, or survival related to segregation of sexes into winter habitats of different quality (Townsend et al. 2009).
Reproductive Success
Clutch size is generally 3-4 eggs. Of 171 Bicknell’s Thrush nests monitored in Vermont from 1993–2007, 48% were successful in fledging at least one chick; the average number of young fledged per nest ranged from 1.5 – 2.1 (McFarland et al. 2008). The major cause of nest failure (accounting for 75% of failed nests) was depredation of eggs or chicks. Rates of nest predation are strongly linked to a widespread masting cycle (often biennial) in montane coniferous forests, in which alternating years of high cone crops result in high Red Squirrel (Tamiasciurus hudsonicus) populations during the following spring and summer. Squirrels are a major nest predator of Bicknell’s Thrush and other open-cup nesting birds (Wallace 1939, Townsend et al. 2015). In years following low autumn cone yields, spring and summer squirrel populations are invariably reduced, and Bicknell’s Thrush nesting success is markedly higher, relative to years following high autumn cone yields when squirrels are abundant (Townsend et al. 2015). The species’ demographic response to this pulsed resource system, which can deviate from a strictly biennial pattern (especially in recent years), needs to be considered in the evaluation of population data.
Life Span and Survivorship
The species’ longevity record, based on band returns at a Vermont breeding site, is of an 11-year-old male and an 8-year-old female (Townsend et al. 2015). A banded male recaptured in Cape Breton in 2009 was at least seven years old (BSC/EOC, unpubl. data). Using mark-recapture analysis, the annual apparent survival estimate of adult birds captured at Vermont breeding sites was 65%, independent of year or sex (Rimmer et al. 2004). In Québec, male annual survival was higher (63%) than female survival (28%) (COSEWIC 2009).
Survival rates of juveniles are poorly known and difficult to assess due to apparent natal dispersal: 6% of fledglings and 19% of independent juveniles banded in Vermont from 1992–2004 returned to their natal mountain (Townsend et al. 2015). Like other songbirds, survival rates of juvenile Bicknell’s Thrush are probably low. On Mt. Mansfield, Vermont in 2000, only 18% of radio-tagged fledglings were known to have survived beyond 30 days (VCE, unpubl. data).
The annual apparent survival rate of individuals captured at a montane broadleaf forest site in the Sierra de Bahoruco, Dominican Republic, from 1994–1999, was 73% (Townsend et al. 2015). Raw recapture rates for Bicknell’s Thrush in the Dominican Republic, however, were much lower than for birds banded at breeding sites in Vermont (28% vs. 65%; VCE unpubl. data).
Population Status
Estimated Population Size
The first estimate of the global population size of Bicknell’s Thrush was made in the early 1990s, when Tony Erskine (CWS) estimated that only 5,000 breeding pairs existed in North America (Nixon 1999). Since then, extensive fieldwork across the breeding range has helped to refine population estimates for the species.
Campbell and Stewart (2012) estimated population size of Bicknell’s Thrush in New Brunswick and Nova Scotia using bird densities estimated by the BSC/EOC High Elevation Landbird Program along with the model of potential Bicknell’s Thrush habitat (VCE, unpubl. data) and the New Brunswick and the Nova Scotia forest inventories. In New Brunswick, the population was estimated to be 2,851 individuals, with 95% lower and upper confidence limits of 1,137 and 10,652 individuals. In Nova Scotia, the estimate was 3,845 individuals, with 95% confidence limits of 1,823 and 7,049 individuals.
In the White Mountains of New Hampshire, satellite imagery and a digital elevation model, coupled with point-count data, was used to model the distribution and abundance of Bicknell’s Thrush (Hale 2006). Spatially explicit predictions of probability of thrush presence were made for each 28.5 × 28.5 m-pixel covering the 70,000 ha study area. Transforming probabilities into relative abundance produced an estimated 4,900 Bicknell’s Thrushes across the study area.
More recently, range-wide data on densities and models of potential habitat have been used to develop an improved global population estimate. Applying region-specific density data (derived from point count surveys) to a model of potential habitat (VCE, unpubl. data) yielded estimates of 57,000 to 77,000 Bicknell’s Thrushes in the U.S. and 40,570 to 49,258 individuals in Canada (COSEWIC 2009), resulting in a global population of 97,570 to 126,258 birds. The Partners in Flight Population Estimates Database version 2.0 estimated the global population of Bicknell’s Thrush at 110,500 individuals (Partners in Flight Science Committee 2013). Finally, Hill and Lloyd (unpublished manuscript) used data collected from 2011-2016 under MBW to estimate a 2016 U.S. population size of 71,618 (95% credible interval: 56,788 – 90,219) Bicknell’s Thrushes.
Population Trends
High-elevation songbird monitoring programs generally indicate declining populations of Bicknell’s Thrush, especially in core and northern parts of the breeding range. The most comprehensive trend data to date are derived from surveys (point counts) conducted by volunteers and field technicians across the northeastern U.S. as part of the Mountain Birdwatch program, in the White Mountain National Forest (WMNF) of New Hampshire and Maine (King et al. 2008), and in the Maritime Provinces (High Elevation Landbird Program). Government and university researchers have monitored sites in Québec since the late 1990s (Y. Aubry, CWS, unpubl. data), although no formal survey program is in place. Bicknell’s Thrush is poorly represented on survey routes of the North American Breeding Bird Survey (BBS); however, data are available from 16 Canadian BBS routes.
The following trend summaries are derived from these aural surveys and represent the best available trend estimates for breeding Bicknell’s Thrush.
United States
- 7% annual decline in the WMNF during 1993–2003 based on WMNF High Elevation Bird Survey program (King et al. 2008, Lambert et al. 2008).
- Significant overall decline from 1989-2010 in an analysis combining several sources of data, including Mountain Birdwatch and WMNF High Elevation Bird Survey program (Ralston et al. 2015), although decline apparently driven by results from the WMNF High Elevation Bird Survey
- No overall trend nor any state-specific trend from 2003–2010 based on Mountain Birdwatch data (Ralston et al. 2015).
- Local extinction of breeding population on Mt Greylock, Massachusetts.
Canada
- 11.5% annual decline in New Brunswick from 2002-2011 (Campbell and Stewart 2012).
- No annual trend detected in Nova Scotia from 2002–2011, likely because there were too few survey routes (Campbell and Stewart 2012).
- Local extinction of several breeding populations in Nova Scotia and New Brunswick (see Breeding Range, above);
- Detected in 35% fewer survey blocks in the northwestern and central highlands of New Brunswick and in 32% fewer squares in the Cape Breton Highlands during the 2nd Maritimes Breeding Bird Atlas (2006-2010) compared to the first Atlas (1986-1990) (Whittam 2015).
- During the Second Breeding Bird Atlas of Québec (2010-2014), detected in less than 50% of the squares occupied during the first Quebec breeding bird atlas (1984-1989), with most of the disappearances occurring in the Gaspé peninsula, at the northern end of the species distribution (Quebec Breeding Bird Atlas, unpubl. data).
- 29% decrease in probability of occupancy at Mont Gosford, Québec from 2001–2007, with no change in detection probability (Y. Aubry, CWS, unpubl. data).
- 60% decline in abundance at Mont Gosford, Québec, from 2001–2007 (Y. Aubry, CWS, unpubl. data).
- 9% annual decline in abundance across Canada (BBS) from 1966–2008 (P. Blancher, Environment Canada, unpubl. data).
During the winter from 1995-2010, a standardized array of 30–35 mist nets were operated at two remote sites in Parque nacional Sierra de Bahoruco in southwestern Dominican Republic (Lloyd et al. 2016). The sites consist of montane cloud forest at 1,775–1,850 m elevation and were separated by 2.6 km of contiguous forest. There was no evidence for temporal trends in capture rate for Bicknell’s Thrush (Lloyd et al. 2016).
Legal Status
Federal and Global Status
- Threatened, Species at Risk Act (Canada).
- Species of continental conservation concern, Partners in Flight (Rosenberg et al. 2016).
- Vulnerable, International Union of Conservation of Nature (BirdLife International 2012).
- N2B in Canada (nationally imperiled) and N4B (nationally apparently secure; last updated 1997) in the U.S., NatureServe Conservation Status (NatureServe 2015).
- Red List. Audubon Watchlist (Butcher et al. 2007)
- Red Watch List – Continental. State of the Birds 2014. (Rosenberg et al. 2014)
- Bird Species of National Concern, U.S. Fish and Wildlife Service (USFWS).
- Petition review for listing under the U.S. Endangered Species Act, USFWS.
- Endangered – Red List. República Dominicana (Ministerio de Medio Ambiente y Recursos Naturales de la República Dominicana 2011).
Partners in Flight Status
The PIF Species Assessment Process evaluates six biological vulnerability factors. Based on a set of carefully defined thresholds, each factor is assigned a score ranging from 1 (to reflect very low concern or importance) to 5 (to reflect the highest concern or importance). Scores for Bicknell’s Thrush were: Population Size (4), Breeding Distribution (4), Non-breeding Distribution (4), Threats to Breeding (3), Threats to Non-breeding (5), and Population Trend (4) for a total Continental Concern score of 17, making it a Tri-National Concern Species, U.S.-Canada Concern Species, Partners in Flight Watch List 2016, and a U.S.-Canada Stewardship Species.
State and Provincial Status (U.S. and Canada)
- Species of Special Concern in Maine, New York, and Vermont
- Species of Special Concern Category B (Responsibility Species) in New Hampshire
- Endangered under the Nova Scotia Endangered Species Act
- Threatened in New Brunswick under the New Brunswick Species at Risk Act
- Vulnerable in Québec, Loi sur les espèces menacées ou vulnérables du Québec
Bicknell’s Thrush has been assigned the following rankings by state and provincial Natural Heritage Programs (NatureServe 2015):
- S1S2B (Critically Imperiled to imperiled) in Nova Scotia
- S2B (Imperiled) in Vermont
- S2S3B (Imperiled to vulnerable) in New Brunswick, New Hampshire, and New York
- S3B (Vulnerable) in Maine and Québec.
- SXB (Presumed extirpated) in Massachusetts.
Associated Species

The endemic and endangered La Selle Thrush (Turdus swalesi) co-occurs with Bicknell’s Thrush on Hispaniola.
Conservation actions aimed at Bicknell’s Thrush are likely to benefit co-occurring species, many of which are also conservation priorities.
To identify co-occurring species of conservation concern, we first queried the IUCN Red List of Threatened Species database to identify Red List species found on islands where Bicknell’s Thrush winters (Cuba, Jamaica, Hispaniola, Puerto Rico) and in the general forest type where it is often found (subtropical/tropical moist forest). A total of 240 plant and animal species were found: 31 plants, 6 insects, 148 reptiles and amphibians, 43 birds, and 12 mammals (Table 1.1).
Table 1.1. IUCN Red List species that occur on islands where Bicknell’s Thrush winters (Cuba, Jamaica, Hispaniola, Puerto Rico) in the same potential habitat (subtropical/tropical moist forest).| Class | Extinct | Critically Endangered | Endangered | Vulnerable | Near Threatened | Total |
|---|---|---|---|---|---|---|
| Jungermanniopsida | 1 | 1 | 2 | |||
| Cycadopsida | 1 | 1 | 1 | 3 | ||
| Pinopsida | 2 | 5 | 2 | 9 | ||
| Liliopsida | 1 | 1 | 2 | 4 | ||
| Magnoliopsida | 4 | 6 | 3 | 13 | ||
| Insecta | 5 | 1 | 6 | |||
| Amphibia | 57 | 51 | 19 | 7 | 134 | |
| Reptilia | 2 | 5 | 1 | 6 | 14 | |
| Aves | 1 | 5 | 10 | 13 | 14 | 43 |
| Mammalia | 2 | 3 | 5 | 2 | 12 | |
| Total | 3 | 72 | 87 | 47 | 31 | 240 |
We next searched State Wildlife Action plans from the U.S. and found 19 bird species that were both identified as Species of Greatest Conservation Need and are found in Bicknell’s Thrush winter (8 species) and/or breeding (11 species) habitat (Table 1.2).
Table 1.2. Bird species occurring in Bicknell’s Thrush habitat that were identified as Species of Greatest Conservation Need in the 2005 State Wildlife Action Plans.| Common Name | Scientific Name | Bicknell's Thrush Habitat |
|---|---|---|
| American Three-toed Woodpecker | Picoides dorsalis | Breeding |
| Bay-breasted Warbler | Setophaga castanea | Breeding |
| Black-backed Woodpecker | Picoides arcticus | Breeding |
| Blackpoll Warbler | Setophaga striata | Breeding |
| Cape May Warbler | Setophaga tigrina | Breeding |
| Gray Jay | Perisoreus canadensis | Breeding |
| Olive-sided Flycatcher | Contopus cooperi | Breeding |
| Purple Finch | Carpodacus purpureus | Breeding |
| Red Crossbill | Loxia curvirostra | Breeding |
| Spruce Grouse | Falcipennis canadensis | Breeding |
| Tennessee Warbler | Vermivora peregrina | Breeding |
| American Redstart | Setophaga ruticilla | Winter |
| Black-and-white Warbler | Mniotilta varia | Winter |
| Black-throated Blue Warbler | Setophaga caerulescens | Winter |
| Hooded Warbler | Wilsonia citrina | Winter |
| Louisiana Waterthrush | Seiurus motacilla | Winter |
| Ovenbird | Seiurus aurocapilla | Winter |
| Swainson's Warbler | Limnothlypis swainsonii | Winter |
| Worm-eating Warbler | Helmitheros vermivorus | Winter |
We also compiled a list of endemic bird species and those at risk that occupy the same winter habitat as Bicknell’s Thrush from the IUCN Red List and the 2005 Puerto Rico Wildlife Action Plan (Table 1.3). Of the 61 species we listed, 56 were endemic species or subspecies. The bulk of the species were from Hispaniola (43%), followed by Puerto Rico (28%), Jamaica (16%), and Cuba (13%).
Table 1.3. Endemic or resident bird species at risk that may be found in Bicknell’s Thrush winter habitat. The list was compiled from the IUCN Red List and the Puerto Rico Wildlife Action Plan.| Species | Scientific Name | Island | IUCN Status | Endemic? |
|---|---|---|---|---|
| Plain Pigeon | Patagioenas inornata | all | NT | N |
| White-crowned Pigeon | Patagioenas leucocephala | all | NT | N |
| Bare-legged Owl | Otus lawrencii | Cuba | Y | |
| Cuban Green Woodpecker | Xiphidiopicus percussus | Cuba | Y | |
| Cuban Pygmy-Owl | Glaucidium siju | Cuba | Y | |
| Cuban Solitaire | Myadestes elisabeth | Cuba | NT | Y |
| Cuban Tody | Todus multicolor | Cuba | Y | |
| Cuban Trogon | Priotelus temnurus | Cuba | Y | |
| Cuban Vireo | Vireo gundlachii | Cuba | Y | |
| Oriente Warbler | Teretistris fornsi | Cuba | Y | |
| Antillean Euphonia | Euphonia musica musica | Hispaniola | Y subspecies | |
| Bananaquit | Coereba flaveola bananivora | Hispaniola | Y subspecies | |
| Black-crowned Palm-Tanager | Phaenicophilus palmarum | Hispaniola | Y | |
| Broad-billed Tody | Todus subulatus | Hispaniola | Y | |
| Eastern Chat-Tanager | Calyptophilus frugivorus | Hispaniola | VU | Y |
| Gray-crowned Palm-Tanager | Phaenicophilus poliocephalus | Hispaniola | NT | Y |
| Greater Antillean Bullfinch | Loxigilla violacea affinis | Hispaniola | Y subspecies | |
| Greater Antillean Elaenia | Elaenia fallax cherriei | Hispaniola | Y subspecies | |
| Green-tailed Ground-Tanager | Microligea palustris | Hispaniola | Y | |
| Hispaniolan Crossbill | Loxia megaplaga | Hispaniola | EN | Y |
| Hispaniolan Emerald | Chlorostilbon swainsonii | Hispaniola | Y | |
| Hispaniolan Highland-Tanager | Xenoligea montana | Hispaniola | VU | Y |
| Hispaniolan Parakeet | Aratinga chloroptera | Hispaniola | VU | Y |
| Hispaniolan Parrot | Amazona ventralis | Hispaniola | VU | Y |
| Hispaniolan Pewee | Contopus hispaniolensis | Hispaniola | Y | |
| Hispaniolan Quail-Dove | Geotrygon leucometopia | Hispaniola | VU | Y |
| Hispaniolan Spindalis | Spindalis dominicensis | Hispaniola | Y | |
| Hispaniolan Trogon | Priotelus roseigaster | Hispaniola | NT | Y |
| Hispaniolan Woodpecker | Melanerpes striatus | Hispaniola | Y | |
| La Selle Thrush | Turdus swalesi | Hispaniola | EN | Y |
| Narrow-billed Tody | Todus angustirostris | Hispaniola | Y | |
| Rufous-throated Solitaire | Myadestes genibarbis montanus | Hispaniola | Y subspecies | |
| Sharp-shinned Hawk | Accipiter striatus striatus | Hispaniola | Y subspecies | |
| Western Chat-Tanager | Calyptophilus tertius | Hispaniola | VU | Y |
| Black-capped Petrel | Pterodroma hasitata | Hispaniola, Cuba, Jamaica | EN | N |
| Golden Swallow | Tachycineta euchrysea | Hispaniola, formerly Jamaica | VU | Y subspecies |
| Black-billed Parrot | Amazona agilis | Jamaica | VU | Y |
| Blue Mountain Vireo | Vireo osburni | Jamaica | NT | Y |
| Crested Quail-dove | Geotrygon versicolor | Jamaica | NT | Y |
| Jamaican Blackbird | Nesopsar nigerrimus | Jamaica | EN | Y |
| Jamaican Parakeet | Eupsittula nana | Jamaica | NT | Y |
| Jamaican Petrel | Pterodroma caribbaea | Jamaica | CR | Y |
| Ring-tailed Pigeon | Patagioenas caribaea | Jamaica | VU | Y |
| Yellow-billed Parrot | Amazona collaria | Jamaica | VU | Y |
| Antillean Euphonia | Euphonia musica sclateri | Puerto Rico | Y subspecies | |
| Elfin Wood Warbler | Setophaga angelae | Puerto Rico | VU | Y |
| Green-throated Carib | Eulampis holosericeus | Puerto Rico | N | |
| Puerto Rican Bullfinch | Loxigilla portoricensis | Puerto Rico | Y | |
| Puerto Rican Emerald | Chlorostilbon maugaeus | Puerto Rico | Y | |
| Puerto Rican Flycatcher | Myiarchus antillarum | Puerto Rico | Y | |
| Puerto Rican Lizard-cuckoo | Saurothera vieilloti | Puerto Rico | Y | |
| Puerto Rican Parrot | Amazona vittata | Puerto Rico | CR | Y |
| Puerto Rican Pewee | Contopus portoricensis | Puerto Rico | Y | |
| Puerto Rican Screech-owl | Megascops nudipes | Puerto Rico | Y | |
| Puerto Rican Stripe-headed Tanager | Spindalis portoricensis | Puerto Rico | Y | |
| Puerto Rican Tanager | Nesospingus speculiferus | Puerto Rico | Y | |
| Puerto Rican Tody | Todus mexicanus | Puerto Rico | Y | |
| Puerto Rican Vireo | Vireo latimeri | Puerto Rico | Y | |
| Puerto Rican Woodpecker | Melanerpes portoricensis | Puerto Rico | Y | |
| Ruddy Quail Dove | Geotrygon montana | Puerto Rico | N | |
| Sharp Shinned Hawk | Accipiter striatus venator | Puerto Rico | Y subspecies |
Chapter 2. The International Bicknell's Thrush Conservation Group
Background
In response to range-wide conservation concerns for Bicknell’s Thrush, VCE and BSC/EOC convened a coalition with a common interest in setting priorities for research and conservation needs for this species. Composed of scientists, natural-resource managers, and conservation planners, this group, the IBTCG, is flexible and inclusive, with no requirement for membership beyond a shared interest in advancing Bicknell’s Thrush conservation. The IBTCG’s overarching goal is to develop a broad, scientifically sound approach to conservation of Bicknell’s Thrush, in order to prevent further declines and to increase current populations to a sustainable level. The group aims to address threats to Bicknell’s Thrush throughout its full life cycle.
The administrative structure of IBTCG consists of a coordination committee, working groups, and members. The role of the coordination committee is to oversee implementation of the Conservation Action Plan (“the Plan”), seek funding, maintain momentum, set meetings and agendas, and identify next steps. Various working groups are formed when needed to facilitate specific activities and maintain momentum towards a desired outcome.
The IBTCG held its inaugural meeting in Woodstock, Vermont in November 2007. The 25 meeting participants included representatives from academia; federal, state, and provincial wildlife agencies; and non-governmental organizations. Five northeastern states and two Canadian provinces were represented. Much of the inaugural meeting of the IBTCG focused on identifying potential limiting factors and corresponding conservation actions that would form the backbone of a Bicknell’s Thrush conservation action plan. Subsequent annual meetings in Hadley, Massachusetts (October 2008) and Québec City, Québec (September 2009), as well as specific working group meetings, focused on further developing priority conservation actions identified during this process and refining early drafts of the conservation action plan.
 The first Plan for Bicknell’s Thrush was published in July 2010 in three languages (English, French, and Spanish). The Plan established a course of conservation and research that was designed to boost the worldwide Bicknell’s Thrush population. Actions included:
The first Plan for Bicknell’s Thrush was published in July 2010 in three languages (English, French, and Spanish). The Plan established a course of conservation and research that was designed to boost the worldwide Bicknell’s Thrush population. Actions included:
- Partner with timber companies and managers of public lands in North America to develop and implement best management practices for breeding habitat.
- Conduct scientific research to monitor and predict the impacts of climate change on Bicknell’s Thrush habitat.
- Improve the protection of currently occupied winter habitat and develop management plans for key forested areas on Hispaniola, including restoration of degraded habitats.
- Strengthen links with local partners in the Caribbean and expand funding for on-the-ground conservation projects throughout the winter range.
The IBTCG held its fourth annual meeting, in conjunction with the Black-capped Petrel Working Group, in Santo Domingo, Dominican Republic on 2-4 November 2010. Sixty conservation biologists from seven countries working in three languages spent three days focused on increasing cooperation between Caribbean and North American IBTCG partners and implementing the Plan (English, Spanish).
On November 4-5, 2015, eight years after the inaugural meeting, 22 members of the IBTCG met at the Marsh-Billings-Rockefeller National Historical Park in Woodstock, Vermont to initiate the process of preparing this revised Plan.
With members spanning the hemisphere, communication and information sharing are critical to the success of this group. The IBTCG developed a website to serve as an accessible clearinghouse for information related to Bicknell’s Thrush conservation and to publicize the group’s activities. The website hosts a summary of Bicknell’s Thrush research and a growing bibliography of relevant publications and reports, including the Plan.
Mission
To develop a broad-based, scientifically sound approach to conserve Bicknell’s Thrush, incorporating research, monitoring, and on-the-ground management actions.
Chapter 3. Conservation goals
The overall conservation goals for Bicknell’s Thrush established by the IBTCG are:
- Increase population size by 25% between 2011 and 2060.
- Maintain or increase the extent of breeding occurrence above 2010 levels.
These goals reflect the two key demographic changes that have increased the vulnerability of Bicknell’s Thrush to extinction and resulted in its listing as a Threatened species by the IUCN (BirdLife International 2012) and the Government of Canada (2012): declines in population size (fewer individuals overall) and local extinctions (Bicknell’s Thrush have disappeared from some parts of their range) (see Chapter 1).
Historic estimates of population size are lacking and recent trends are inconsistent across the breeding range (Ralston et al. 2015); nonetheless, annual declines over the past two decades of 2-7% in parts of the U.S. (Lambert et al. 2008, Ralston et al. 2015) and 9-20% in Canada (COSEWIC 2009) suggest that current population size is substantially depressed. The extent of occurrence during the breeding season has shrunk over the same time period due to local extinctions of populations in Massachusetts, Québec, New Brunswick, and Nova Scotia (Townsend et al. 2015, Whittam 2015).
We cannot directly evaluate progress on these goals since the release of the first Plan, and indeed both goals are best appraised on relatively long (e.g., decadal) time scales. However, we continue to believe that maintaining or recovering populations at the periphery of the species’ range, for example the Cape Breton Highlands of Nova Scotia, is achievable. We also continue to believe that the goal of offsetting recent population losses by effecting a 25% increase in population size is attainable, although it will require instituting widespread and effective habitat management, protection, and restoration efforts across the breeding and wintering ranges within the next five years. The actions identified in this plan should guide these efforts, and should be implemented immediately. Delays in implementation may jeopardize the timeline for reaching conservation goals given the time lag between the onset of habitat management or restoration and the consequent change in population dynamics of Bicknell’s Thrush.
Chapter 4. Population threats and conservation actions
Numerous anthropogenic threats affect Bicknell’s Thrush and collectively these threats exert strong regulatory effects on the population growth rate. Several demographic and ecological characteristics of Bicknell’s Thrush also act synergistically with anthropogenic threats to further increase vulnerability to extinction. For example, the small global population size, clumped distribution of habitat at breeding and wintering sites, apparent limited natal dispersal, skewed adult sex ratio, and potential winter habitat sex-segregation are all characteristics that increase vulnerability to threats or stochastic events.
In this chapter, we detail threats to Bicknell’s Thrush and appropriate actions to mitigate those threats that were identified by the IBTCG during the 2015 meeting. We also rank the relative risk posed by each threat (it’s timing, scope, and severity). Threats, threat scores, and actions all follow the IUCN classification scheme. Threat scores are based on the expert opinion of Plan contributors (see Appendix A for more details on methodology). A summary of threats, threat scores, actions, and a justification for the assigned scores is found in the Threats and Action table.
High-impact threats are those that are ongoing, affect a large percentage of the population, and are capable of producing steep and rapid declines in numbers within the affected population. High-priority actions are those that address high-impact threats, are highly feasible, and have direct effects on the threat.
Although high-impact threats should be prioritized when implementing conservation actions, medium-impact threats should not be ignored. The cumulative effect of the many medium-impact threats may pose as great a risk to the viability of Bicknell’s Thrush as the few high-impact threats, and thus mitigating medium-impact threats is an important component of achieving the overall goals of the revised Plan. Medium-impact threats may also emerge as priorities for action by actors with a regional focus (e.g., state or provincial agencies or NGOs whose mission is local or regional in scope) because many of the medium-impact threats are severe (i.e., likely to produce significant declines) even if their scope is limited. The cost-effectiveness of the actions to address threats must also be considered; for example, if a relatively low investment leads to a large reduction in a medium-impact threat, this may be more cost-effective than if the same investment leads to only a minimal reduction in a high-impact threat.
The following sections summarize the high- and medium-impact threats and actions that can be taken to mitigate them. All of the threats identified by IBTCG and its members, including low-impact threats and threats of uncertain impact, are listed in their entirety at the end of this chapter. Key stakeholders—that is, the governmental or non-governmental entities with responsibility for implementing conservation actions—are identified in the list.
Summary of high-impact threats and high-priority actions
The three most significant threats to the viability of Bicknell’s Thrush are the conversion of forest habitat to agriculture and pasture on Hispaniola, incompatible forestry practices on the breeding grounds, and loss of habitat due to climate change. All three threats result in a direct loss of habitat, albeit at different time scales. Conversion of forest to agriculture and pasture and incompatible forestry practices are acute threats, whereas habitat loss due to climate change is apt to occur slowly. Actions that address these threats directly and that are likely to succeed are the highest priorities for implementation during the lifespan of the revised Plan.
Forest clearing for agriculture on the wintering grounds
In keeping with the IUCN scheme for classifying conservation threats, we recognize as distinct threats the loss of forest for large-scale agricultural production and the loss of forest for smaller-scale farming. Agro-industrial farms clear large areas of forest to produce relatively high-value crops, often for export. An example is the ongoing clearing of montane forest in Parque Nacional Sierra de Bahoruco to create fields for avocado plantations that will supply foreign markets. Small-holder farming is practiced at a smaller spatial scale and relies primarily on family labor, although the aggregated effect can be large.
Both types of agricultural threats can be addressed by a similar suite of actions. High-priority actions to counter the threat posed by agro-industrial farming and small-holder farming include two that directly target the integrity of existing protected areas that are threatened by agricultural expansion: stronger enforcement of protected-area boundaries and training to increase institutional capacity at all levels for management of protected areas.
Most potential habitat for Bicknell’s Thrush is not within the bounds of existing protected areas (McFarland et al. 2013) and thus protection of potential habitat on private or otherwise unprotected property should be pursued through purchase or easement agreements. In many cases, this will require clarifying land-tenure claims.
Effectively using market mechanisms is a high-priority action to reduce incentives for pursuing agro-industrial farming within Bicknell’s Thrush habitat. This may include disincentivizing industrial farming within protected areas or other important thrush habitat through boycotts or consumer-education efforts and incentivizing sustainable agricultural practices (i.e., those conducted outside of protected areas). Reserva Zorzal, in the Domincan Republic’s Cordillera Septentrional, provides an example of the latter. Seventy percent of the land is set aside for reforestation so that it will eventually support Bicknell’s Thrush; a smaller percentage is used to grow cacao, which generates revenue and jobs.
Direct economic incentives, for example cash payments, or other positive incentives such as technical assistance in adopting agricultural activities that avoid destroying habitat for Bicknell’s Thrush, are a high-priority action that could be targeted at small-holders. A pilot project testing this approach is currently underway in Haiti (J. Goetz, pers. comm.). As is the case with outright protection of land through purchase or easement, provision of direct payments to abandon incompatible land uses will require clarifying land-tenure claims.
Developing livelihood alternatives should also be a high priority. This might include developing and promoting enterprises that are linked to intact forest ecosystems, such as ecotourism.
Although not a high-priority action because its effects on the threat are indirect, research and monitoring to evaluate the efficacy of these different conservation interventions should be conducted in tandem with their implementation.
Incompatible forestry practices on the breeding grounds
Bicknell’s Thrush breeding habitat is characterized by dense, short-statured forest stands dominated by balsam fir. As such, some forest-management activities can create breeding habitat, for example when the removal of mature canopy trees during harvest creates conditions that allow the development of dense stands of fir saplings. At the same time, some forest-management activities can destroy or degrade breeding habitat. In particular, any silvicultural practice that reduces stem density in otherwise suitable forest stands, for example pre-commercial thinning, poses a potential threat. Other activities associated with forest management, for example road building, may also eliminate suitable habitat and cause direct mortality. In Canada, incompatible forestry practices have been addressed through government regulation and the development of BMPs. In the U.S., efforts have focused on developing BMPs (Lambert et al. 2017).
High-priority actions to address this threat focus on continuing to:
- Implement best-management practices for forestry operations, and
- Implement policies that require avoiding or limiting use of pre-commercial thinning in Bicknell’s Thrush habitat.
An important action, although of lower priority because its effects on the threat are indirect, is research to better understand the distribution of Bicknell’s Thrush in managed forests in the U.S. Large areas of managed forest exist in Maine and New Hampshire that may provide habitat for Bicknell’s Thrush, although no comprehensive surveys have been conducted to determine the extent to which stands in these forests are used for nesting. A better understanding of how Bicknell’s Thrush use managed forests would offer insights into the silvicultural systems and intermediate treatments that could be used to create breeding habitat in forests managed for wood and pulp production. Other important research questions include determining whether retaining patches of unthinned habitat can promote persistence of Bicknell’s Thrush in landscapes where pre-commercial thinning is used, determining whether Bicknell’s Thrush occupy and reproduce successfully in thinned stands that have regrown to the point of canopy closure, and determining the area of unthinned habitat necessary to meet conservation goals.
Climate change
Models predict that most or all breeding habitat for Bicknell’s Thrush is at risk from climate change due to the sensitivity of balsam fir to increased temperature (Rodenhouse et al. 2007, Dombroskie et al. 2010, Irfan Ashraf et al. 2015, Iverson et al. 2016, Boulanger et al. 2016). Hardwood forests, which are not habitable by Bicknell’s Thrush, are expected to expand in response to climate change and replace conifer forests, potentially resulting in significant loss of breeding habitat. Wintering habitat is also threatened by climate change, in particular the expected increases in aridity across the Greater Antilles (Neelin et al. 2006, Rauscher et al. 2008, Zelazowski et al. 2011). Less precipitation and increasing frequency of drought may directly affect forest structure and composition and may also increase the frequency of forest loss due to wildfire.
No priority actions were identified for this threat because the actions to address this threat are indirect, and include supporting policies that reduce the atmospheric concentration of greenhouse gases, research that refines predicted effects of climate change on habitat, research that identifies areas that may act as climate-change refugia, and research that addresses whether forest management could increase resistance of montane forests to climate change.
Summary of medium-impact threats and priority actions to address them
Threats scored as imposing a medium impact on Bicknell’s Thrush were generally either those that affected most of the global population but caused, or were likely to cause, slow declines, or those that caused very steep declines in a small percentage of the global population. As such, medium-impact threats may pose significant conservation challenges at local or regional scales. Implementing actions that address medium-impact threats, especially those with higher scores, is an important part of achieving the goals of the revised Plan; conservation activities should not focus exclusively on high-priority threats.
Wind-energy development on the breeding grounds
Creation of wind-energy facilities can result in the permanent loss of habitat and therefore represents a threat of medium to high severity. The overall extent of breeding habitat lost to wind-energy development is believed modest at present, although some regions – such as Québec – have seen more extensive impacts. The impact of wind-energy development on the wintering grounds is unknown, but may merit further investigation (see Suspended threats and threats of uncertain timing, scope, or severity). This threat could pose a greater risk in the future on both the breeding and wintering grounds if overlap between Bicknell’s Thrush habitat and areas desirable for wind-energy generation is high and if regulatory and economic factors continue to encourage wind-energy development.
The priority actions for this threat include implementing policies that require application of the mitigation hierarchy (avoid, minimize, or compensate) for any impacts to potential or actual breeding habitat and developing best-management practices for construction, operation, and maintenance of wind-energy facilities. For example, in Québec, wind-energy developments that are located in potential habitat of Bicknell’s Thrush must conduct surveys prior to the establishment of roads and turbines (MDDEFP 2013). Depending on whether the species is present, and the overall quality of habitat in the area, mitigation actions may be required.
Shifting agriculture in wintering habitat
In keeping with the IUCN scheme for classifying conservation threats, we recognize the threat posed by shifting agriculture as distinct from that posed by other forms of agriculture (see Forest clearing for agriculture on the wintering grounds, above). Shifting agriculture, or slash-and-burn agriculture, is a small-scale agricultural system that provides one or a few crop rotations on a field before abandonment. Shifting agriculture involves forest clearing and burning and thus contributes to habitat loss and fragmentation. An example of this threat is the sharecropping system on the southern slope of Sierra de Bahoruco. Although this form of agriculture is as destructive of habitat as small-holder and agro-industrial farming, at present the scope of the population at risk from this threat appears relatively small. This threat could pose a greater risk in the future if this system of agriculture continues to expand into habitat for Bicknell’s Thrush.
The priority actions that address this threat are the same as those for small-holder farming:
- Offer livelihood alternatives,
- Protect habitat through easements or purchase,
- Offer direct payments for conservation,
- Enforce protected-area boundaries, and
- Improve institutional capacity for protected-area management.
Charcoal production in wintering habitat
Single-tree/small-group harvest for charcoal production results in the degradation and loss of wintering habitat. This threat appears localized but is likely to cause fairly rapid declines in areas where production is extensive.
Priority actions that address this threat include:
- Enforce protected-area boundaries,
- Improve institutional capacity for protected-area management, and
- Provide alternative fuels.
Invasive species in wintering habitat
Invasive, exotic mammals including feral pigs, rats, cats, and mongoose are widespread in forests that provide habitat to Bicknell’s Thrush during the winter. Rats, cats, and mongoose cause direct mortality whereas feral pigs diminish habitat quality via the disturbance they cause to understory vegetation during foraging. The threat posed by these mammals is likely chronic and sufficient to produce measurable changes in population size. No priority actions were identified for this threat due to the infeasibility of implementing effective, large-scale control of invasive mammals.
Mining
Expansion of existing larimar and bauxite mines in the Dominican Republic, or reactivation of defunct mines, has the potential to result in forest loss and a further decline in the availability of wintering habitat. Mining may pose threats to Bicknell’s Thrush habitat in Cuba and Jamaica, but more information is needed to determine the extent and scope of the threat. Priority actions to address this threat include reclamation and restoration of defunct mines by mining companies and implementation of policies that require mitigation for any forest loss caused by new mining activity.
Communication towers
Erecting communications towers is an ongoing activity that can result in the permanent loss of breeding habitat and can cause direct mortality when birds collide with towers. As such, communications towers represent a threat of medium to high severity. The scope of the threat at present is modest and tends to be concentrated in certain locations. Actions to address this threat include development of BMPs for construction and maintenance of communication towers and access roads located in potential habitat for Bicknell’s Thrush and implementation of policies that require application of the standard mitigation hierarchy. Many of the BMPs for wind-energy development (MDDEFP 2013) may be applicable to communication towers. In the U.S., the Federal Aviation Administration has issued new guidelines that prohibit the use of non-flashing lights on new towers >150 feet tall, and has requested that owners of existing towers develop plans to bring them into compliance (see https://www.fcc.gov/general/tower-and-antenna-siting). These changes are expected to reduce the incidence of collision.
Altered fire regimes on the wintering grounds
The frequency and extent of forest fires in some winter areas may be increasing and institutional capacity to respond is limited. Broadleaf montane forests are not fire-adapted, but may be increasingly at risk if climate change results in more frequent and more intense drought as predicted by climate models. Repeated fire can convert broadleaf forest to pine forest and thus reduce the amount of wintering habitat for Bicknell’s Thrush (Myers et al. 2004). Actions to address this threat include training and deploying additional wildland firefighters and adopting fire-management strategies that reduce the risk of large, intense fires, especially at the pine/broadleaf forest ecotone.
Excessive browsing by moose
Browsing by unusually large populations of moose is preventing forest regeneration in Cape Breton Highlands and favoring grassy clearings over dense stands of regenerating balsam fir that would provide habitat for Bicknell’s Thrush (Smith et al. 2010). This threat may cause local declines or extirpations. An ongoing experimental cull program and Before-After/Control-Impact study is underway that is aimed at addressing this threat (Greg Campbell, pers. comm.).
Acid precipitation
Acid precipitation damaged high-elevation red spruce forests in the U.S. (DeHayes et al. 1999) and may have degraded the quality of breeding habitat for Bicknell’s Thrush either directly by reducing calcium availability needed for reproduction, or indirectly by changing habitat structure. Acid precipitation may also increase the availability of the methylated form of mercury, a known toxicant. Evidence indicates that emissions controls required by the U.S. Clean Air Act have led to reductions in acid deposition and recovery of degraded forest soils (Burns et al. 2011, Lawrence et al. 2015), suggesting that the threat posed by acid precipitation is diminishing. However, complete recovery of affected ecosystems will require further reductions in emissions (Burns et al. 2011). Supporting policies that lead to further reductions in emissions of sulfur dioxide and nitrogen oxide therefore would presumably benefit Bicknell’s Thrush.
Threats and Actions
The following information is summarized in the Threats and Actions table.
High-impact Threats
Threat: Annual and perennial non-timber crops - small-holder agriculture.
Small-scale agriculture based on permanently maintained plots. When forest suitable for Bicknell’s Thrush is cleared for small-holder farming, it represents a source of habitat loss. Continued operation of existing farms precludes habitat restoration. The trend in area under small-holder farms is unknown.
- Where: Wintering
- Threat level: 8/High
- Timing: Continuing (3)
- Scope: Affects the majority of the population (2)
- Severity: Causing or likely to cause very rapid declines (>30% over 10 years or three generations) (3)
Action: Land/water protection – site/area protection.
- Acquire private properties that provide habitat for Bicknell’s Thrush.
- Feasibility: Likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Government that have regulatory authority over protected areas that support Bicknell’s Thrush.
- NGOs
- Private companies
Action: Land/water management – site/area management.
- Increased enforcement of protected-area regulations.
- Feasibility: Likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Government that have regulatory authority over protected areas that support Bicknell’s Thrush.
- NGOs
- Demarcate protected-area boundaries.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Government agencies that have regulatory authority over protected areas that support Bicknell’s Thrush.
- NGOs
Action: Education & awareness – Training
- Improve institutional capacity and accountability for management of protected areas by training park staff. Where protected areas are co-managed, training should also seek to improve capacity of staff of the co-managing organization.
- Feasibility: Likely
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- NGOs
- Government agencies that have regulatory authority over protected areas that support Bicknell’s Thrush.
Action: Livelihood, economic & other incentives – Linked enterprises & livelihood alternatives
- Enhance opportunities for local ecotourism ventures; promote sustainable, permanent shade-grown crops like cacao or coffee on degraded lands as a buffer to intact Bicknell’s Thrush habitat.
- Feasibility: Unlikely
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- Government agencies that have regulatory authority over protected areas that support Bicknell’s Thrush.
- Government agencies that have regulatory authority over commerce, tourism, or agricultural development.
- NGOs
- Private companies
Action: Livelihood, economic & other incentives – Conservation payments
- Direct payments to landholders to protect and/or allow recovery of Bicknell’s Thrush habitat.
- Feasibility: Likely
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- NGOs
Action: Research – Actions
- Research on drivers of land-use change and effectiveness of different interventions.
- Feasibility: Extremely likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
- Research how to restore abandoned agricultural fields and develop best practices for habitat restoration.
- Feasibility: Extremely likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Threat: Annual and perennial non-timber crops - agro-industrial farming.
Crops grown at an industrial scale, often for export; notably cacao, coffee, and avocado. May include associated impacts from small-holder farming used by workers. When forest suitable for Bicknell’s Thrush is cleared for agro-industry farming, it represents a source of habitat loss. Continued operation of existing farms precludes habitat restoration. Most notable current example is avocado farming within cloud forest of Parque Nacional Sierra de Bahoruco, Dominican Republic.
- Where: Wintering
- Threat level: 7/High
- Timing: ongoing (3)
- Scope: Affects the minority of the population (1)
- Severity: Causing or likely to cause very rapid declines (>30% over 10 years or three generations) (3)
Action: Land/water protection – site/area protection.
- Acquire private properties that provide habitat for Bicknell’s Thrush.
- Feasibility: Likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Government agencies
- NGOs
- Private companies
Action: Land/water management – site/area management.
- Demarcate protected-area boundaries.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Government agencies that have regulatory authority over protected areas that support Bicknell’s Thrush.
- NGOs
- Increased enforcement of protected-area regulations.
- Feasibility: Likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Government that have regulatory authority over protected areas that support Bicknell’s Thrush.
- NGOs
Action: Education & awareness – Training
- Improve institutional capacity for management of protected areas by training park staff. Where protected areas are co-managed, training should also seek to improve capacity of staff of the co-managing organization.
- Feasibility: Likely
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- NGOs
- Government agencies that have regulatory authority over protected areas that support Bicknell’s Thrush.
Action: Livelihood, economic & other incentives – Linked enterprises & livelihood alternatives
- Enhance opportunities for local ecotourism ventures; promote sustainable, permanent shade-grown crops like cacao or coffee especially on degraded lands as a buffer to intact Bicknell’s Thrush habitat.
- Feasibility: Unlikely
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- Government agencies that have regulatory authority over protected areas that support Bicknell’s Thrush.
- Government agencies that have regulatory authority over commerce, tourism, or agricultural development.
- NGOs
- Private companies
Action: Livelihood, economic & other incentives – Market forces
- Promote boycotts of crops grown within protected areas, certification of crops grown using sustainable practices (e.g., forest set-asides within plantations).
- Feasibility: Likely
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- NGOs
Action: Livelihood, economic & other incentives – Conservation payments
- Direct payments to landholders to protect and/or allow recovery of Bicknell’s Thrush habitat.
- Feasibility: Likely
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- NGOs
- Direct payments to land tenants with legal title or possessory interests within designated protected areas to relinquish property.
- Feasibility: Likely
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- Government agencies that have regulatory authority over protected areas that support Bicknell’s Thrush.
- NGOs
Action: Research – Actions
- Research on drivers of land-use change and effectiveness of different interventions.
- Feasibility: Extremely likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
- Research how to restore abandoned agricultural fields and develop best practices for habitat restoration.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGO
Threat: Biological resource use - logging and wood harvesting, unintentional effects: large scale
Incompatible forestry practices render forests unsuitable for nesting.
- Where: Breeding
- Threat level: 7/High
- Timing: Continuing (3)
- Scope: Affects the minority of the population (1)
- Severity: Causing or likely to cause very rapid declines (3)
- Threat level: 7/High
Action: Law & policy – Private sector standards & codes
- Implement best management practices for forestry operations.
- Feasibility: Extremely likely
- Effect on threat: Direct
- Responsible stakeholders:
- Federal, state, and provincial agencies that have regulatory authority over migratory songbirds or forestry operations.
- Forestry companies
- Private landholders
- NGOs
Action: Law & policy – Policies & regulations
- Implement policies that require avoiding or limiting use of pre-commercial thinning in Bicknell’s Thrush habitat and promote compliance with existing rules and BMPs.
- Feasibility: Extremely likely
- Effect on threat: Direct
- Responsible stakeholders:
- Federal, state, and provincial agencies that have regulatory authority over migratory songbirds or forestry operations.
- NGOs
Action: Research – Population size, distribution & trends
- Research distribution of Bicknell’s Thrush in industrial forests of U.S. and Canada.
- Feasibility: Extremely Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
- Determine whether retaining patches of unthinned habitat can promote persistence of Bicknell’s Thrush in landscapes where pre-commercial thinning is used.
- Feasibility: Extremely Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
- Forestry companies
- Determine whether Bicknell’s Thrush occupy and reproduce successfully in thinned stands that have regrown to the point of canopy closure.
- Feasibility: Extremely Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
- Forestry companies
- Determine the area of unthinned habitat necessary to meet conservation goals.
- Feasibility: Extremely Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
- Forestry companies
- Determine at what point of maturity a suitable stand becomes no longer favourable to nesting.
- Feasibility: Extremely Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
- Forestry companies
Threat: Climate change and severe weather - habitat shifting and alteration
Future climate in much of the current breeding and wintering distribution may become unsuitable for the forest types inhabited by Bicknell’s Thrush. Climate models predict that spruce-fir forests will recede in the U.S. and southern Canada, and that forests in the Caribbean will experience substantially more arid conditions in the future.
- Where: Breeding and Wintering
- Threat level: 7/High
- Timing: Continuing (3)
- Scope: Affects the whole population (3)
- Severity: Causing or likely to cause relatively slow, but significant, declines (1)
Action: Research – Threats
- Research effects of forecast changes in climate on distribution of breeding habitat for Bicknell’s Thrush.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Action: Research – Actions
- Identify areas of currently suitable habitat that may be resistant to climate change and that may act as refugia.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
- Research whether forest management can increase resistance of balsam-fir forests to climate change.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- Federal, state, or provincial agencies that have regulatory or management authority over potential habitat for Bicknell’s Thrush.
- NGOs
Medium-impact threats
Threat: Energy production and mining - renewable energy
Wind-energy facilities and associated infrastructure that destroy habitat.
- Where: Breeding
- Threat level: 6/Medium
- Timing: ongoing (3)
- Scope: Affects a negligible proportion of the population (0)
- Severity: Causing or likely to cause very rapid declines (3)
- Threat level: 6/Medium
Action: Law & policy – Policies & regulations
- Implement policies that require application of the mitigation hierarchy (avoid, minimize, or compensate) for any impacts to potential or actual habitat.
- Feasibility: Extremely Likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Government agencies that have regulatory authority over land-use and/or Bicknell’s Thrush
- Government agencies that have regulatory authority over energy and/or infrastructure development
- NGOs
Action: Research – Threats
- Research effects of wind-energy facilities and associated infrastructure.
- Feasibility: Extremely Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Action: Law & policy – Private sector standards & codes
- Develop best management practices for construction, operation, and maintenance of wind-energy facilities.
- Feasibility: Extremely Likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- NGOs, industry
Threat: Annual and perennial non-timber crops - shifting agriculture
Relatively small-scale agricultural systems that provide one or a few crop rotations before abandonment. Usually involves forest clearing and burning and thus contributes to habitat loss and fragmentation.
- Where: Wintering
- Threat level: 6/Medium
- Timing: ongoing (3)
- Scope: Affects a negligible proportion of the population (0)
- Severity: Causing or likely to cause very rapid declines (>30% over 10 years or three generations) (3)
- Threat level: 6/Medium
Action: Land/water protection – site/area protection.
- Acquire private properties that provide habitat for Bicknell’s Thrush.
- Feasibility: Likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Government that have regulatory authority over protected areas that support Bicknell’s Thrush.
- NGOs
- Private companies
Action: Land/water management – site/area management
- Increased enforcement of protected-area regulations.
- Feasibility: Likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Government that have regulatory authority over protected areas that support Bicknell’s Thrush.
- NGOs
- Demarcate protected-area boundaries.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Government agencies that have regulatory authority over protected areas that support Bicknell’s Thrush.
- NGOs
Action: Education & awareness – Training
- Improve institutional capacity for management of protected areas by training park staff. Where protected areas are co-managed, training should also seek to improve capacity of staff of the co-managing organization.
- Feasibility: Likely
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- NGOs
- Government agencies that have regulatory authority over protected areas that support Bicknell’s Thrush.
Action: Livelihood, economic & other incentives – Linked enterprises & livelihood alternatives
- Enhance opportunities for local ecotourism ventures; promote sustainable, permanent shade-grown crops like cacao or coffee, especially on degraded lands as a buffer to intact Bicknell’s Thrush habitat.
- Feasibility: Unlikely
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- Government agencies that have regulatory authority over protected areas that support Bicknell’s Thrush.
- Government agencies that have regulatory authority over commerce, tourism, or agricultural development.
- NGOs
Action: Livelihood, economic & other incentives – Conservation payments
- Direct payments to landholders to protect and/or allow recovery of Bicknell’s Thrush habitat.
- Feasibility: Likely
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- NGOs
- Direct payments to land tenants with legal title or possessory interests within designated protected areas to relinquish property.
- Feasibility: Likely
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- Government agencies that have regulatory authority over protected areas that support Bicknell’s Thrush.
- NGOs
Action: Research – Actions
- Research on drivers of land-use change and effectiveness of different interventions.
- Feasibility: Extremely likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
- Research how to restore abandoned agricultural fields and develop best practices for habitat restoration.
- Feasibility: Extremely Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Threat: Biological resource use - logging and wood harvesting - unintentional effects: subsistence/small scale
Single-tree/small-group harvest for charcoal and lime production results in degradation and loss of wintering habitat.
- Where: Wintering
- Threat level: 6/Medium
- Timing: Continuing (3)
- Scope: Affects the minority of the population (1)
- Severity: Causing or likely to cause rapid declines (2)
- Threat level: 6/Medium
Action: Land/water management – site/area management
- Increased enforcement of protected-area regulations.
- Feasibility: Likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Government agencies that have regulatory authority over protected areas that support Bicknell’s Thrush.
- NGOs
- Demarcate protected-area boundaries.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Government agencies that have regulatory authority over protected areas that support Bicknell’s Thrush.
- NGOs
Action: Education & awareness – Training
- Improve institutional capacity for management of protected areas by training park staff. Where protected areas are co-managed, training should also seek to improve capacity of staff of the co-managing organization.
- Feasibility: Likely
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- NGOs
- Government agencies that have regulatory authority over protected areas that support Bicknell’s Thrush.
Action: Livelihood, economic & other incentives – Substitution
- Provide alternative fuels.
- Feasibility: Likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Government agencies
- NGOs
- Private companies
Action: Research – Actions
- Research how to restore abandoned charcoal production areas and develop best practices for habitat restoration.
- Feasibility: Extremely Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Action: Law & policy – Private sector standards & codes
- Develop and implement best management practices for sustainable charcoal harvest.
- Feasibility: Unlikely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- NGOs
- Government agencies
Threat: Invasive and other problematic species, genes and diseases - invasive non-native/alien species/disease
Invasive animals, especially pigs, disturb forest understory and may diminish quality of winter habitat. Also, introduced predators such as cats, rats, and mongoose directly increase mortality.
- Where: Wintering
- Threat level: 6/Medium
- Timing: Continuing (3)
- Scope: Affects the majority of the population (2)
- Severity: Causing or likely to cause fluctuations (1)
- Threat level: 6/Medium
Action: Land/water management – invasive/problematic species control
- Where appropriate, implement programs to reduce numbers of invasive animals.
- Feasibility: Extremely unlikely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Government that have regulatory authority over protected areas that support Bicknell’s Thrush.
- NGOs
Action: Research – Actions
- Research effectiveness of control measures.
- Feasibility: Extremely unlikely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Threat: Energy production and mining - mining and quarrying
Potential for expansion of mining and mining infrastructure (i.e. access roads) may result in habitat loss.
- Where: Wintering
- Threat level: 5/Medium
- Timing: future (2)
- Scope: Affects a negligible proportion of the population (0)
- Severity: Causing or likely to cause very rapid declines (3)
- Threat level: 5/Medium
Action: Land/water management – habitat & natural process restoration
- Reclaim and restore areas degraded by open-pit mining operations.
- Feasibility: Unlikely
- Effect on threat: Direct
- Responsible stakeholders:
- Mining companies
- Government agencies that have regulatory authority over mining activities
Action: Law & policy – Policies & regulations
- Create and Implement policies that require application of the mitigation hierarchy (avoid, minimize, or compensate) for any impacts to potential or actual wintering habitat.
- Feasibility: Unlikely
- Effect on threat: Direct
- Responsible stakeholders:
- Government agencies that have regulatory authority over mining activities
Threat: Transportation and service corridors - utility and service lines
Communication towers and associated infrastructure result in loss of high-elevation forests on breeding grounds.
- Where: Breeding
- Threat level: 5/Medium
- Timing: Continuing (3)
- Scope: Affects a negligible proportion of the population (0)
- Severity: Causing or likely to cause rapid declines (2)
- Threat level: 5/Medium
Action: Law & policy – Policies & regulations
- Implement policies that require application of the mitigation hierarchy (avoid, minimize, or compensate) for any impacts to potential or actual breeding habitat.
- Feasibility: Extremely likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Federal, state or provincial agencies that have regulatory authority over communications infrastructure.
Action: Law & policy – Private sector standards & codes
- Develop and implement best management practices for construction, operation, and maintenance of communication towers and associated infrastructure.
- Feasibility: Extremely likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Federal, state or provincial agencies that have regulatory authority over communications infrastructure.
- Industry
Threat: Fire and fire suppression - increase in fire frequency/intensity
Lack of resources to control fire and potential increase in fire frequency on the wintering grounds due to climate change and human use of forest.
- Where: Wintering
- Threat level: 5/Medium
- Timing: Continuing (3)
- Scope: Affects the minority of the population (1)
- Severity: Causing or likely to cause relatively slow, but significant, declines (1)
- Threat level: 5/Medium
Action: Education & awareness – Training
- Increase number of firefighters trained in wildland fire suppression in protected areas and communities
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Government agencies responsible for wildland fire suppression.
Action: Education & awareness – Awareness & communications
- Conduct wildland fire education in communities near vulnerable protected areas.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Government agencies responsible for wildland fire suppression.
- NGOs
Action: Land/water management – site/area management
- Assess available equipment for wildland fire suppression and facilitate equipment acquisition where needed.
- Feasibility: Likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Government agencies responsible for wildland fire suppression.
- NGOs
Action: Land/water management – habitat & natural process restoration
- Adopt fire-management practices and plans that reduce risk of large, intense fires, especially at pine/broadleaf forest ecotone.
- Feasibility: Likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Government agencies responsible for wildland fire suppression.
- NGOs
Threat: Invasive and other problematic species, genes, and diseases - problematic native species/diseases - named species
Large moose populations in Cape Breton Highlands, Nova Scotia are converting forested areas once suitable for Bicknell’s Thrush into grassy clearings.
- Where: Breeding (Nova Scotia)
- Threat level: 5/Medium
- Timing: Continuing (3)
- Scope: Affects a negligible proportion of the population (0)
- Severity: Causing or likely to cause rapid declines (2)
- Threat level: 5/Medium
Action: Land/water management – invasive/problematic species control
- Continue experimental moose culling program.
- Feasibility: Likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Parks Canada
- NGOs
Action: Research – Actions
- Research effectiveness of control measures.
- Feasibility: Extremely likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Threat: Pollution - airborne pollutants - acid rain
Acid precipitation harms spruce trees, thus potentially degrading breeding habitat, and depletes soil calcium, which may causes a decline in reproductive success.
- Where: Breeding
- Threat level: 4/Medium
- Timing: Continuing (3)
- Scope: Affects the minority of the population (1)
- Severity: Causing or likely to cause negligible declines (0)
- Threat level: 4/Medium
Action: Law & policy – Policies & regulations
- Support policies that lead to reduced emissions of sulfur dioxide and nitrogen oxide.
- Feasibility: Unlikely
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- NGOs
Action: Research – Threats
- Research effects of acid precipitation on Bicknell’s Thrush.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Low-impact threats
Threat: Residential and commercial development - tourism and recreation areas - Ski Areas
Ski-area construction or expansion—including off-piste glading—that destroys breeding habitat.
- Where: Breeding
- Threat level: 3/Low
- Timing: ongoing (3)
- Scope: Affects a negligible proportion of the population (0)
- Severity: Causing or likely to cause negligible declines (0)
- Threat level: 3/Low
Action: Law & policy – Policies & regulations
- Implement policies that require application of the mitigation hierarchy (avoid, minimize, or compensate) for any impacts to potential or actual breeding habitat.
- Feasibility: Extremely Likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Federal, state, or provincial agencies that have regulatory authority over potential habitat for Bicknell’s Thrush.
- Industry
Action: Law & policy – Private sector standards & codes
- Work with ski areas and user groups to implement existing best management practices.
- Feasibility: Extremely likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- NGOs
- Industry groups (e.g., ski-area associations)
- User groups (e.g., trail associations, backcountry-skiing associations)
Threat: Agriculture and aquaculture - wood and pulp plantations - agro-industry plantations
Reforesting cleared areas with quick-growing exotic softwoods for carbon markets. Although not a net loss of habitat, represents an opportunity cost because that area remains uninhabitable by Bicknell’s Thrush.
- Where: Wintering
- Threat level: 3/Low
- Timing: ongoing (3)
- Scope: Affects a negligible proportion of the population (0)
- Severity: Causing or likely to cause negligible declines (0)
- Threat level: 3/Low
Action: Law & policy – Policies & regulations
- Implement policies that prohibit use of exotic species in reforestation efforts within areas that could support habitat for Bicknell’s Thrush.
- Feasibility: Unknown
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- NGOs
- Government agencies that have regulatory authority over protected areas that support Bicknell’s Thrush
- Government agencies that have regulatory authority over forestry activities
Action: Research – Actions
- Identify suitable native species for use in restoration and research horticultural requirements.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Action: Education & awareness – Training
- Train forest agents to transfer knowledge to users about research on reforestation with native species.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
- Government agencies activities
Action: Law & policy – Private sector standards & codes
- Encourage adoption of standards for carbon offsets that promote use of native species.
- Feasibility: Likely
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- NGOs
Threat: Biological resource use - logging and wood harvesting - unintentional effects: large scale (breeding)
Forestry activities conducted in suitable habitat during the breeding season may inadvertently cause mortality of eggs or nestlings.
- Where: Breeding
- Threat level: 3/Low
- Timing: Continuing (3)
- Scope: Affects the minority of the population (0)
- Severity: Causing or likely to cause negligible declines (0)
- Threat level: 3/Low
Action: Law & policy – Private sector standards & codes
- Develop and implement best management practices for forestry operations.
- Feasibility: Extremely likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- NGOs
Action: Law & policy – Policies & regulations
- Implement policies that require conducting forestry operations that might result in inadvertent mortality outside of Bicknell’s Thrush breeding season when these operations occur in Bicknell’s Thrush habitat.
- Feasibility: Extremely likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Federal, state, or provincial agencies that have regulatory authority over forestry operations.
Threat: Human intrusion and disturbance - recreational activities
Recreational activities such as hiking or birdwatching in breeding habitat may disturb individual birds and reduce reproductive success.
- Where: Breeding
- Threat level: 3/Low
- Timing: Continuing (3)
- Scope: Affects a negligible proportion of the population (0)
- Severity: Causing or likely to cause negligible declines (0)
- Threat level: 3/Low
Action: Education & awareness – Awareness & communications
- Outreach campaigns to recreational user groups about Bicknell’s Thrush and its habitat.
- Feasibility: Extremely Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- NGOs
Threat: Human intrusion and disturbance - work and other activities
Mortality during species research.
- Where: Breeding
- Threat level: 3/Low
- Timing: Continuing (3)
- Scope: Affects a negligible proportion of the population (0)
- Severity: Causing or likely to cause negligible declines (0)
- Threat level: 3/Low
Action: Education & awareness – Training
- Adhere to the Ornithological Council’s Guidelines for the Use of Wild Birds in Research.
- Feasibility: Extremely Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs engaged in research
Suspended threats and threats of uncertain timing, scope, or severity
Threat: Residential and commercial development - tourism and recreation areas - migration
Development of coastal habitat used for stopover during migration. Geolocator studies indicate that Bicknell’s Thrush regularly stop during migration in coastal North Carolina and possibly in the Bahamas and Cuba. Development in these areas, which we assume would most likely come in the form of tourist resorts, might pose a threat although we do not have any evidence that this is currently happening nor can we estimate the number of birds potentially affected or the severity of the threat posed.
- Where: Migration
- Threat level: Unknown
- Timing: Unknown
- Scope: Unknown
- Severity: Unknown
- Threat level: Unknown
Action: Research – Life history & ecology
- Identify stopover sites and habitat use during stopover.
- Feasibility: Extremely Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Action: Law & policy – Policies & regulations
- Implement policies that require application of the mitigation hierarchy (avoid, minimize, or compensate) for any impacts to stopover habitat.
- Feasibility: Likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Government agencies that have regulatory authority over potential habitat for Bicknell’s Thrush.
Threat: Residential and commercial development - tourism and recreation areas - wintering
Development of resorts leads to loss of wintering habitat. The possibility of resort development was raised during the IBTCG meeting, but we have no other information on this threat.
- Where: Wintering
- Threat level: Unknown
- Timing: Unknown
- Scope: Unknown
- Severity: Unknown
- Threat level: Unknown
Action: Research – Threats
- Conduct a risk analysis of wintering habitat to identify areas at risk of land-use/land-cover change and the drivers of that change.
- Feasibility: Extremely Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Threat: Livestock farming and ranching
Livestock grazing in protected areas in Haiti and Dominican Republic diminish habitat quality for Bicknell’s Thrush.
- Where: Wintering
- Threat level: 5/Medium
- Timing: ongoing (3)
- Scope: Unknown
- Severity: Causing or likely to cause negligible declines (0)
- Threat level: 5/Medium
Action: Land/water management – site/area management
- Increased enforcement of protected-area regulations.
- Feasibility: Likely
- Effect on threat: Direct, immediate
- Responsible stakeholders:
- Government that have regulatory authority over protected areas that support Bicknell’s Thrush.
- NGOs
- Demarcate protected-area boundaries.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Government agencies that have regulatory authority over protected areas that support Bicknell’s Thrush.
- NGOs
Action: Research – Threats
- Research extent of threat posed by livestock use of Bicknell’s Thrush habitat in protected areas.
- Feasibility: Extremely Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Threat: Energy production and mining - renewable energy (wind)
Wind-energy facilities and associated infrastructure that destroy stopover habitat or wintering habitat or kill individuals during migration. Collisions with wind turbines during migration is a possible risk, but we were unaware of any data that would allow us to identify the scope or severity of the threat. We are unaware of any existing or planned wind-energy facilities in wintering habitat, although we recognize that the potential for such developments exists. We had no information that would allow us to identify the scope or severity of the threat.
- Where: Migration, Wintering
- Threat level: Unknown
- Timing: Unknown
- Scope: Unknown
- Severity: Unknown
- Threat level: Unknown
Action: Research – Threats
- Research effects of wind-energy facilities and associated infrastructure (e.g., mortality from collisions, impacts to stopover or wintering habitat).
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
- Private companies
Threat: Transportation and service corridors - utility and service lines - communications towers
Erecting communications towers can result in the permanent loss of montane forest used as wintering habitat. Most bird species are vulnerable to mortality caused by collision with communication towers, and we assume that this includes Bicknell’s Thrush. However, we are unaware of any data that would allow us to identify the timing, scope, or severity of this threat during either migration or wintering.
- Where: Migration, Wintering
- Threat level: Unknown
- Timing: Unknown
- Scope: Unknown
- Severity: Unknown
- Threat level: Unknown
Action: Research – Threats
- Research effects of communication towers and associated infrastructure (e.g., mortality from collisions, impacts to stopover habitat or wintering habitat).
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
- Private companies
Threat: Natural system modification - other ecosystem modifications
Reduction in anthropogenic (logging) and natural (fire, insect outbreaks) disturbances that create suitable breeding habitat may reduce the extent of occurrence of Bicknell’s Thrush and limit population size. This threat was identified in the first Plan. The global financial crisis that began in 2008 resulted in depressed commodities markets, which raised concerns about the viability of the timber industry in Canada and the State of Maine. Breeding habitat for Bicknell’s Thrush is maintained and created by periodic disturbance and the absence of regular timber harvest could lead to a reduction in the amount of habitat available in some parts of the breeding range. Natural disturbances may be less frequent now than historically. However, timber continues to be a viable industry and spruce budworm is epidemic in parts of the breeding range at present and thus we considered this threat to be suspended. As such, we do not identify actions for this threat.
- Where: Breeding
- Threat level: Unknown
- Timing: Now suspended (could come back in the long term) (1)
- Scope: Unknown
- Severity: Unknown
- Threat level: Unknown
Threat: Invasive and other problematic species, genes, and diseases - problematic native species/diseases - Comb Forkedfern
Empirical evidence and anecdotal observation indicate that the Comb Forkedfern (Dicranopteris pectinata) inhibits forest regeneration in the Dominican Republic. As such, it may hinder habitat restoration and thus limit recovery of Bicknell’s Thrush populations. However, we could not find information that allowed us to score the scope or severity of the threat.
- Where: Wintering (D.R.)
- Threat level: Unknown
- Timing: Continuing (3)
- Scope: Unknown
- Severity: Unknown
- Threat level: Unknown
Action: Research – Threats
- Research extent of threat posed by Dicranopteris pectinata.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Action: Habitat and natural process restoration
- Where needed based on findings of preceding action, implement restoration techniques identified by Slocum et al. (2006).
- Feasibility: Likely
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- Academic/government scientists
- Government agencies that have regulatory authority over protected areas that support Bicknell’s Thrush.
- Government agencies that have regulatory authority over commerce, tourism, or agricultural development.
- NGOs
Threat: Pollution - agricultural and forestry effluents - herbicides and pesticides
Effects on non-target species of spraying for mosquito control in wintering areas may have unintended consequences for Bicknell’s Thrush. This issue was raised by the IBTCG, but we could find no further information about the timing, scope, or severity of this threat.
- Where: Wintering
- Threat level: Unknown
- Timing: Unknown
- Scope: Unknown
- Severity: Unknown
- Threat level: Unknown
Action: Research – Threats
- Research consequences of mosquito control on Bicknell’s Thrush.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Threat: Pollution - airborne pollutants (lead)
Lead present in montane forest soils may have sub-lethal physiological effects on Bicknell’s Thrush. This issue was raised in the first Plan, but we could find no further information about the timing, scope, or severity of this threat.
- Where: Breeding, Wintering
- Threat level: Unknown
- Timing: Unknown
- Scope: Unknown
- Severity: Unknown
- Threat level: Unknown
Action: Research – Threats
- Research extent of lead exposure and consequences for Bicknell’s Thrush.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Threat: Pollution - airborne pollutants (mercury)
Mercury from coal burning and waste incineration bioaccumulates at successive trophic levels and is known to occur at elevated levels in blood of Bicknell’s Thrush, possibly causing sub-lethal physiological or behavioral effects. Mercury occurs at elevated levels in blood of Bicknell’s Thrush, and individuals are exposed to mercury on both wintering and breeding grounds, but we have no information about the consequences of exposure on individual vital rates or population growth rate.
- Where: Breeding, Wintering
- Threat level: Unknown
- Timing: Ongoing (3)
- Scope: Affects the majority of the population (2)
- Severity: Unknown
- Threat level: Unknown
Action: Research – Threats
- Research consequences of mercury exposure on Bicknell’s Thrush.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Action: Law & policy – Policies & regulations
- Support policies that lead to reduced emissions of mercury.
- Feasibility: Unlikely
- Effect on threat: Direct, delayed
- Responsible stakeholders:
- NGOs
Threat: Pollution - airborne pollutants (ozone)
Ground-level ozone can accumulate in the air at high elevations on the breeding range. Exposure to elevated ozone levels has deleterious effects on human health, but whether this poses a threat to Bicknell’s Thrush is unclear and we could find no information addressing effects of ground-level ozone on wildlife populations.
- Where: Breeding
- Threat level: Unknown
- Timing: Unknown
- Scope: Unknown
- Severity: Unknown
- Threat level: Unknown
Action: Research – Threats
- Research consequences for Bicknell’s Thrush of exposure to ground-level ozone.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Threat: Climate change and severe weather - habitat shifting and alteration - predators
Climate change may disrupt an important interaction between red squirrels and Bicknell’s Thrush on the breeding grounds. In a warmer climate, balsam fir may produce cones every year, instead of every other year, and allow large, permanent populations of red squirrel (Tamiasciurus hudsonicus), an important nest predator, to exist in the mountains of the northeastern U.S. and Québec. Predation on eggs and young by red squirrels can cause widespread reproductive failure among Bicknell’s Thrush in some years, and any ecological change that allowed red squirrels to establish large, permanent populations in high-elevation forests within the breeding range could cause steep and rapid declines. However, uncertainty exists concerning the timing of this threat and its scope, especially given anecdotal evidence that populations in other parts of the range coexist with red squirrels.
- Where: Breeding
- Threat level: Unknown
- Timing: Unknown
- Scope: Unknown
- Severity: Causing or likely to cause very rapid declines (3).
- Threat level: Unknown
Action: Research – Threats
- Research whether climate change alters balsam fir cone cycle and red squirrel population dynamics in such a way as to affect Bicknell’s Thrush population dynamics, and whether these processes operate across the breeding range.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Threat: Climate change and severe weather - habitat shifting and alteration - prey phenology
Warmer springs within the breeding range may advance the phenology of important prey species of Bicknell’s Thrush, but not the date at which Bicknell’s Thrush return to the breeding grounds, causing a mismatch between the timing of maximum prey availability and Bicknell’s Thrush nesting. Although studies of other species have implicated phenological mismatches as a cause of steep and rapid declines (Both et al. 2006, Møller et al. 2008, Jones and Cresswell 2010), other studies have shown that mismatched phenology does not always result in population-level impacts (Reed et al. 2013). This suggests the consequences of changing phenology may be species-specific, and no information is available regarding this phenomenon in Bicknell’s Thrush. Additional research is needed to clarify the risk posed by this threat.
- Where: Breeding
- Threat level: Unknown
- Timing: Unknown
- Scope: Unknown
- Severity: Unknown
- Threat level: Unknown
Action: Research – Threats
- Research whether phenological mismatch has consequences for population dynamics.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Threat: Climate change and severe weather - habitat shifting and alteration - competitors
Climate change may disrupt a potentially important interaction between Swainson’s Thrush (Catharus ustulatus) and Bicknell’s Thrush on the breeding grounds. Overlap in breeding habitat of Swainson’s Thrush, which may be a superior competitor, and Bicknell’s Thrush is thought to be limited by lower tolerance to cold in Swainson’s Thrush. Climate change may allow greater degree of overlap and potential for increased interspecific competition. However, empirical documentations of competition between the species, and evidence of population-level impacts to Bicknell’s Thrush, are lacking. Indeed, in parts of Canada, the two species coexist without any evident negative consequences for Bicknell’s Thrush.
- Where: Breeding
- Threat level: Unknown
- Timing: Unknown
- Scope: Unknown
- Severity: Unknown
- Threat level: Unknown
Action: Research – Threats
- Research whether competition with Swainson’s Thrush has fitness consequences for Bicknell’s Thrush and whether degree of overlap between two species is increasing.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Threat: Climate change and severe weather - habitat shifting and alteration - forest pests and pathogens
Forest pests, such as balsam wooly adelgid (Insecta: Adelges piceae), normally limited by cold climate may expand into montane forests and lead to loss and degradation of breeding habitat. Forests in the southern portion of Bicknell’s Thrush may be especially vulnerable in the short term to pest infestations that might degrade habitat quality, potentially causing steep declines. However, empirical information on the timing, scope, and severity of this threat is lacking.
- Where: Breeding
- Threat level: Unknown
- Timing: Unknown
- Scope: Unknown
- Severity: Unknown
- Threat level: Unknown
Action: Research – Threats
- Conduct threat assessment to examine potential impact of forest pests on Bicknell’s Thrush.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Threat: Climate change and severe weather - droughts
Increased frequency and severity of drought throughout the Caribbean Basin may diminish quality of winter habitat and increase risk of forest fire, but additional research is needed to document this phenomenon and quantify its timing, scope, and severity.
- Where: Wintering
- Threat level: Unknown
- Timing: Unknown
- Scope: Unknown
- Severity: Unknown
- Threat level: Unknown
Action: Research – Threats
- Research effects of drought on quality of Bicknell’s Thrush habitat.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Threat: Climate change and severe weather - storms and flooding
Greater frequency of inclement weather, such as heavy rain or wind, during the breeding season may lead to increased nest failure and reduced reproductive success. Documentation of this phenomenon, as well as continued downscaling of climate models, is needed to evaluate the risk posed by changes in the frequency of inclement weather.
- Where: Breeding
- Threat level: Unknown
- Timing: Unknown
- Scope: Unknown
- Severity: Unknown
- Threat level: Unknown
Action: Research – Threats
- Continue monitoring relevant demographic rates in long-term research sites.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Threat: Climate change and severe weather - storms and flooding - migration
Greater frequency of severe storms could cause increased mortality during migration and lead to declines in population size. At present, however, the degree of risk posed by this threat is unknown, and additional research is needed.
- Where: Breeding
- Threat level: Unknown
- Timing: Unknown
- Scope: Unknown
- Severity: Unknown
- Threat level: Unknown
Action: Research – Threats
- Research survival during migration and expand efforts to track birds during migration.
- Feasibility: Likely
- Effect on threat: Indirect
- Responsible stakeholders:
- Academic/government scientists
- NGOs
Chapter 5. Evaluating Success
This revised Plan provides an adaptive framework to guide Bicknell’s Thrush conservation and is intended to be updated regularly. As goals are achieved and information gaps filled, results will be used to refine the Plan and implement effective strategies to increase the population size of Bicknell’s Thrush. The success of the revised Plan should be evaluated on both short- and long-term bases. Long-term success of the revised Plan depends on achieving the population goals established by the IBTCG. Short-term success of the revised Plan can be measured by success in implementing conservation actions identified here. We address both types of evaluation in this chapter.
Evaluating long-term success
Mountain Birdwatch 2.0
Evaluating progress towards the overall population goals—increase population size by 25% between 2011 and 2060 and maintain or increase the extent of breeding occurrence above 2010 levels—requires a rigorous, range-wide monitoring program that will allow estimation of breeding population size and trends at multiple geographic scales. Mountain Birdwatch 2.0 (MBW 2.0) is the program developed and implemented by VCE, BSC/EOC,CWS, USFWS, and WMNF to carry out this monitoring. This survey was designed to be implemented on the breeding grounds, where it is currently most feasible to monitor Bicknell’s Thrush. Breeding birds frequently vocalize and are thus easily detected by aural surveys, and there is greater capacity to conduct large-scale, volunteer-based monitoring programs on the breeding grounds. Hart and Lambert (2008) describe the goals, objectives, and protocols for MBW 2.0.
Mountain Birdwatch 2.0 has been implemented annually in the U.S., New Brunswick, and Nova Scotia since 2011, and must continue on a long-term basis so that population status and trends can be monitored. However, challenges with the implementation of MBW 2.0 in Canada reduce the capacity of the program to monitor any change across the whole breeding range. Whereas routes in the U.S. have met the programmatic goal of achieving a 30% encounter rate (i.e., 30% of surveys yield a detection of a Bicknell’s Thrush), surveys in Canada have not. Encounter rates in Québec, Nova Scotia, and New Brunswick have generally been <10%. The causes of low encounter rate in Canada are not known with certainty, but may include incomplete habitat saturation (e.g., suitable habitat is unoccupied due to small population size of Bicknell’s Thrush), bias associated with the placement of survey routes (e.g., most routes occur only in areas made accessible by the presence of logging roads), and that the habitat model used to define the sampling frame for MBW 2.0 predicts locations only of potential habitat, not actual habitat as influenced by forestry operations.
Irrespective of the cause, low encounter rates in Canada jeopardize the ability of MBW 2.0 to estimate trends in population size with the desired precision. Low encounter rates in Canada also increase the risk that partners abandon the MBW 2.0 monitoring scheme in favor of alternatives that better meet local or regional needs, which would complicate efforts to estimate global trends in population size. Problems with MBW 2.0 must be addressed during the next five years if this effort at coordinated bird monitoring is to persist; if MBW 2.0 cannot be implemented effectively, then an alternative approach for monitoring long-term success of the Plan must be developed.
Evaluating short-term success
The updated IBTCG website will serve as a clearinghouse for information about current and completed projects that address actions identified in the Plan. Project leaders will have the ability to add information to a wiki embedded within the IBTCG website, allowing for real-time updates on success in addressing elements of the Plan.
Chapter 6. Literature Cited
- Adamus, P. R. (1987). Atlas of Breeding Birds in Maine, 1978-1983. Maine Dep. Inland Fish. Wildl., Augusta, p. 176.
- Arendt, W. J. (1992). Status of North American migrant landbirds in the Caribbean region: a summary. In Ecology and conservation of Neotropical migrant landbirds (J. M. Hagan and D. W. Johnston, Editors). Smithson. Inst. Press, Washington, D.C., pp. 143–171.
- Atwood, J. A., C. C. Rimmer, K. P. McFarland, S. H. Tsai, and L. R. Nagy (1996). Distribution of Bicknell’s Thrush in New England and New York. Wilson Bulletin 108:650–661.
- Aubry, Y., A. Desrochers, and G. Seutin (2011). Response of Bicknell’s Thrush (Catharus bicknelli) to boreal silviculture and forest stand edges: a radio-tracking study. Canadian Journal of Zoology 89:474–482.
- BirdLife International (2012). Catharus bicknelli. The IUCN Red List of Threatened Species 2012: e.T22728467A39707077. [Online.] Available at http://www.iucnredlist.org/details/22728467/0. doi: 10.2305/IUCN.UK.2012-1.RLTS.T22728467A39707077.en
- Both, C., S. Bouwhuis, C. M. Lessells, and M. E. Visser (2006). Climate change and population declines in a long-distance migratory bird. Nature 441:81–83.
- Boulanger, Y., A. R. Taylor, D. T. Price, D. Cyr, E. McGarrigle, W. Rammer, G. Sainte-Marie, A. Beaudoin, L. Guindon, and N. Mansuy (2016). Climate change impacts on forest landscapes along the Canadian southern boreal forest transition zone. Landscape Ecology:1–17.
- Burns, D. A., J. A. Lynch, B. J. Cosby, M. E. Fenn, J. S. Baron, and [us Epa Clean Air (2011). National Acid Precipitation Assessment Program report to Congress 2011: An integrated assessment. National Science and Technology Council, Washington, DC.
- Campbell, G., and B. Stewart (2012). High Elevation Landbird Program 10-year report. [Online.] Available at http://www.bsc-eoc.org/library/acbithreport.pdf.
- Chisholm, S. E., and M. L. Leonard (2008). Effect of forest management on a rare habitat specialist, the Bicknell’s Thrush (Catharus bicknelli). Canadian Journal of Zoology 86:217–223.
- Christie, D. (1993). Survey of breeding birds in Fundy National Park, 1992. Volume I: Survey methods and results. Canadian Parks Service Contract no. FNP92-004.
- Connolly, V., G. Seutin, J.-P. L. Savard, and G. Rompre (2002). Habitat use by the Bicknell’s Thrush in the Estrie Region, Quebec. Wilson Bulletin 114:333–341.
- COSEWIC (2009). COSEWIC assessment and status report on the Bicknell’s Thrush Catharus bicknelli in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa.
- DeHayes, D. H., P. G. Schaberg, G. J. Hawley, and G. R. Strimbeck (1999). Acid rain impacts on calcium nutrition and forest health: alteration of membrane-associated calcium leads to membrane destabilization and foliar injury in red spruce. Bioscience 49:789–800.
- Dombroskie, S., M. McKendy, C. Ruelland, W. Richards, C. P.-A. Bourque, and F.-R. Meng (2010). Assessing impact of projected future climate on tree species growth and yield: development of an evaluation strategy. Mitigation and Adaptation Strategies for Global Change 15:307–320.
- Erskine, A. J. (1992). Atlas of breeding birds of the Maritime Provinces. Nimbus Publishing Ltd. and Nova Scotia Museum, Halifax.
- Evans, W. R. (1994). Nocturnal flight call of Bicknell’s Thrush. Wilson Bulletin 106:55–61.
- Garrido, O. H., and F. Garcia Montaña (1975). Catálogo de las aves de Cuba. Academia de Ciencias de Cuba, La Habana.
- Goetz, J. E., K. P. Mcfarland, and C. C. Rimmer (2003). Multiple paternity and multiple male feeders in Bicknell’s Thrush (Catharus bicknelli). Auk 120:1044–1053.
- Government of Canada (2012). Order Amending Schedule 1 to the Species at Risk Act. Canada Gazette, Part 1 146:1163–1208.
- Hale, S. R. (2006). Using satellite imagery to model distribution and abundance of Bicknell’s Thrush (Catharus bicknelli) in New Hampshire’s White Mountains. Auk 123:1038–1051.
- Hart, J. A., and J. D. Lambert (2008). Mountain Birdwatch: protocol and standard operating procedures for monitoring high-elevation landbirds in the Northern Appalachians and Laurentian regions. [Online.] Available at https://data.doi.gov/dataset/mountain-birdwatch-protocol-and-standard-operating-procedures-for-monitoring-high-elevatio.
- Irfan Ashraf, M., C. P.-A. Bourque, D. A. MacLean, T. Erdle, and F.-R. Meng (2015). Estimation of potential impacts of climate change on growth and yield of temperate tree species. Mitigation and Adaptation Strategies for Global Change 20:159–178.
- Iverson, L. R., F. R. Thompson, S. Matthews, M. Peters, A. Prasad, W. D. Dijak, J. Fraser, W. J. Wang, B. Hanberry, H. He, M. Janowiak, et al. (2016). Multi-model comparison on the effects of climate change on tree species in the eastern US: results from an enhanced niche model and process-based ecosystem and landscape models. Landscape Ecology:1–20.
- Jones, T., and W. Cresswell (2010). The phenology mismatch hypothesis: are declines of migrant birds linked to uneven global climate change? Journal of Animal Ecology 79:98–108.
- Kibbe, D. P. (1985). Gray-cheeked Thrush. In Breeding Bird Atlas of Vermont (D. P. Kibbe and S. B. Laughlin, Editors). University Press of New England, Hanover, NH, pp. 242–243.
- King, D., J. Lambert, J. Buonaccorsi, and L. Prout (2008). Avian population trends in the vulnerable montane forests of the Northern Appalachians, USA. Biodiversity and Conservation 17:2691–2700.
- Lambert, J. D., D. I. King, J. P. Buonaccorsi, and L. S. Prout (2008). Decline of a New Hampshire Bicknell’s Thrush Population, 1993-2003. Northeastern Naturalist 15:607–618.
- Lambert, J. D., K. P. McFarland, and C. C. Rimmer (2017). Guidelines for managing Bicknell’s thrush habitat in the United States. [Online.] Available at http://bicknellsthrush.org/best-management-practices/.
- Lawrence, G. B., P. W. Hazlett, I. J. Fernandez, R. Ouimet, S. W. Bailey, W. C. Shortle, K. T. Smith, and M. R. Antidormi (2015). Declining acidic deposition begins reversal of forest-soil acidification in the northeastern U.S. and eastern Canada. Environmental Science and Technology 49:13103–13111.
- Leonard, M. L., and S. E. Chisholm (2008). Effect of forest management on a rare habitat specialist, the Bicknell’s Thrush (Catharus bicknelli). Canadian Journal of Zoology 86:217–223.
- Llanes Sosa, A., Y. Aubry, R. Oviedo Prietoi, A. Hernández Marrero, and J. I. Martínez Betancourt (2003). Hábitat utilizados por el Tordo de Bicknell (Catharus bicknelli), durante su residencia invernal en Cuba. Memorias del congreso de Botanica, Ciudad Habana.
- Lloyd, J. D., C. C. Rimmer, and K. P. McFarland (2016). Assessing conservation status of resident and migrant birds on Hispaniola with mist-netting. PeerJ 3:e1541.
- McFarland, K. P., C. C. Rimmer, S. J. K. Frey, S. D. Faccio, and B. B. Collins (2008). Demography, Ecology and Conservation of Bicknell’s Thrush in Vermont, with a Special Focus on the Northeast Highlands. Technical Report 08-03. [Online.] Available at http://www.vtecostudies.org/PDF/VCEBITHReport2008.pdf.
- McKinnon, E. A., H. Askanas, and A. W. Diamond (2014). Nest-patch characteristics of Bicknell’s Thrush in regenerating clearcuts, and implications for precommercial thinning. Northeastern Naturalist 21:259–270.
- MDDEFP (2013). Protocole d’inventaire de la grive de Bicknell et de son habitat. Ministère du Développement durable, de l’Environnement, de la Faune et des Parcs, secteur de la faune, Québec, Canada.
- Ministerio de Medio Ambiente y Recursos Naturales de la República Dominicana (2011). Lista de especies en peligro de extinción, amenazadas o protegidas de la República Dominicana. Santo Domingo de Guzmán, Republica Dominicana.
- Møller, A. P., D. Rubolini, and E. Lehikoinen (2008). Populations of migratory bird species that did not show a phenological response to climate change are declining. Proceedings of the National Academy of Sciences of the United States of America 105:16195–16200.
- Monroe, B. L., R. C. Banks, J. W. Fitzpatrick, T. R. Howell, N. K. Johnson, H. Ouellet, J. V. Remsen, and R. W. Storer (1995). Fortieth Supplement to the American Ornithologists’ Union Check-List of North American Birds. Auk 112:819–830.
- Myers, R., J. O’Brien, D. Mehlman, and C. Bergh (2004). Fire management assessment of the highland ecosystems of the Dominican Republic. GFI publication no. 2004-2. The Nature Conservancy, Arlington, VA.
- NatureServe (2015). Bicknell’s Thrush. NatureServe Explorer: An online encyclopedia of life [web application]. Version 7.1. [Online.] Available at http://explorer.natureserve.org.
- Neelin, J. D., M. Münnich, H. Su, J. E. Meyerson, and C. E. Holloway (2006). Tropical drying trends in global warming models and observations. Proceedings of the National Academy of Sciences of the United States of America 103:6110–6115.
- Nixon, E. (1999). COSEWIC assessment and status report on the Bicknell’s thrush Catharus bicknelli in Canada. In COSEWIC assessment and status report on the Bicknell’s thrush Catharus bicknelli in Canada (COSEWIC, Editor). Committee on the Status of Endangered Wildlife in Canada, Ottawa, pp. 1–43.
- Nixon, E. A., S. B. Holmes, and A. W. Diamond (2001). Bicknell’s Thrushes (Catharus bicknelli) in New Brunswick clear cuts: their habitat associations and co-occurrence with Swainson’s Thrushes (Catharus ustulatus). Wilson Bulletin 113:33–40.
- Ouellet, H. (1993). Bicknell’s thrush: taxonomic status and distribution. The Wilson Bulletin 105:545–571.
- Ouellet, H. (1996). Bicknell’s Thrush. In The Breeding Birds of Quebec (Y. Aubry and J. Gauthier, Editors). Association Québecoise des Groupes d’Ornithologues; Province of Quebec Society for the Protection of Birds; Canadian Wildlife Service, Québec Region, Montréal, pp. 784–787.
- Partners in Flight Science Committee (2013). Population Estimates Database, version 2013. [Online.] Available at http://rmbo.org/pifpopestimates/.
- Peterson, J. (1988). Gray-cheeked Thrush. In The atlas of the breeding birds of New York State. (R. F. Andrle and J. R. Carroll, Editors). Cornell Univ. Press, Ithaca, NY, pp. 320–321.
- Ralston, J., D. I. King, W. V. DeLuca, G. J. Niemi, M. J. Glennon, J. C. Scarl, and J. D. Lambert (2015). Analysis of combined data sets yields trend estimates for vulnerable spruce-fir birds in northern United States. Biological Conservation 187:270–278.
- Rauscher, S. A., F. Giorgi, N. S. Diffenbaugh, and A. Seth (2008). Extension and Intensification of the Meso-American mid-summer drought in the twenty-first century. Climate Dynamics 31:551–571.
- Reed, T. E., V. Grøtan, S. Jenouvrier, B.-E. Sæther, and M. E. Visser (2013). Population growth in a wild bird is buffered against phenological mismatch. Science 340:488–491.
- Richards, T., and C. R. Foss (1994). Bicknell’s Thrush. In Breeding Bird Atlas of New Hampshire (C. R. Foss, Editor). Audubon Soc. New Hampshire, Dover, pp. 218–219.
- Rimmer, C. C., and J. E. Goetz (2010). Avifaunal surveys in La Visite National Park—last vestiges of montane broadleaf forest in eastern Haiti. Journal of Caribbean Ornithology 23:31–43.
- Rimmer, C. C., K. P. McFarland, and J. E. Goetz (1999). Distribution, habitat use, and conservation status of Bicknell’s Thrush in the Dominican Republic. El Pitirre 12:114.
- Rimmer, C. C., K. P. McFarland, J. D. Lambert, and R. B. Renfrew (2004). Evaluating the use of Vermont ski areas by Bicknell’s Thrush: applications for Whiteface Mountain, New York. [Online.] Available at http://www.vtecostudies.org/PDF/ORDA2004.pdf.
- Rimmer, C. C., J. M. Townsend, A. K. Townsend, E. M. Fernández, and J. Almonte (2005). Avian diversity, abundance, and conservation status in the Macaya Biosphere Reserve of Haiti. Ornitologia Neotropical 16:219–230.
- Rodenhouse, N. L., S. N. Matthews, K. P. McFarland, J. D. Lambert, L. R. Iverson, A. Prasad, T. S. Sillett, and R. T. Holmes (2007). Potential effects of climate change on birds of the Northeast. Mitigation and Adaptation Strategies for Global Change 13:517–540.
- Rompre, G., Y. Aubry, and A. Kirkconnell (1999). Notes on some Cuban birds. Cotinga 11:31–33.
- Rompré, G., Y. Aubry, and A. Kirkconnell (2000). Recent observations of threatened birds in Cuba. Cotinga 13:66.
- Rosenberg, K. V., J. A. Kennedy, R. Dettmers, R. P. Ford, D. Reynolds, J. D. Alexander, C. J. Beardmore, P. J. Blancher, R. E. Bogart, G. S. Butcher, A. F. Camfield, et al. (2016). Partners in Flight Landbird Conservation Plan: 2016 Revision for Canada and Continental United States. Partners in Flight Science Committee.
- Rosenberg, K. V., D. Pashley, B. Andres, P. J. Blancher, G. S. Butcher, W. C. Hunter, D. Mehlman, A. O. Panjabi, M. Parr, G. Wallace, and D. Wiedenfeld (2014). The State of the Birds 2014 Watch List. [Online.] Available at http://www.stateofthebirds.org/watch-list.
- Salafsky, N., D. Salzer, A. J. Stattersfield, C. Hilton-Taylor, R. Neugarten, S. H. M. Butchart, B. Collen, N. Cox, L. L. Master, S. O’Connor, and D. Wilkie (2008). A standard lexicon for biodiversity conservation: unified classifications of threats and actions. Conservation Biology 22:897–911.
- Slocum, M. G., T. M. Aide, J. K. Zimmerman, and L. Navarro (2006). A strategy for restoration of montane forest in anthropogenic fern thickets in the Dominican Republic. Restoration Ecology 14:526–536.
- Smith, C., K. Beazley, P. Duinker, and K. A. Harper (2010). The impact of moose (Alces alces andersoni) on forest regeneration following a severe spruce budworm outbreak in the Cape Breton Highlands, Nova Scotia, Canada. Alces 46:135–150.
- Townsend, J., K. P. McFarland, C. C. Rimmer, W. G. Ellison, and J. E. Goetz (2015). Bicknell’s Thrush (Catharus bicknelli). The Birds of North America Online (A. Poole, Ed.). [Online.] Available at http://dx.doi.org/10.2173/bna.592. doi: 10.2173/bna.592
- Townsend, J. M., C. C. Rimmer, and K. P. McFarland (2009). Investigating the limiting factors of a rare, vulnerable species: Bicknell’s Thrush. In Proceedings of the Fourth International Partners in Flight Conference: Tundra to Tropics (T. D. Rich, C. Arizmendi, D. Demarest and C. Thompson, Editors). Partners in Flight, McAllen, Texas, pp. 91–95.
- Townsend, J. M., C. C. Rimmer, A. K. Townsend, and K. P. McFarland (2011). Sex and age ratios of Bicknell’s Thrush wintering in Hispaniola. Wilson Journal of Ornithology 123:367–372.
- Veit, R. R., and W. R. Petersen (1993). Birds of Massachusetts. Massachusetts Audubon Society, Lincoln, Massachusetts.
- Wallace, G. J. (1939). Bicknell’s Thrush, its taxonomy, distribution, and life history. Proceedings of the Boston Society of Natural History 41:211–402.
- Wetmore, A., and B. H. Swales (1931). The Birds of Haiti and the Dominican Republic. Bulletin of the Museum of Comparative Zoology at Harvard College 63:340–341.
- Whittam, B. (2015). Bicknell’s Thrush. In Second Atlas of Breeding Birds of the Maritime Provinces (R. Stewart, K. A. Bredin, A. R. Couturier, A. G. Horn, D. Lepage, S. Makepeace, P. D. Taylor, M.-A. Villard and R. M. Whittam, Editors). Bird Studies Canada, Environment Canada, Natural History Society of Prince Edward Island, Nature New Brunswick, New Brunswick Department of Natural Resources, Nova Scotia Bird Society, Nova Scotia Department of Natural Resources, and Prince Edward Island Department of Agriculture and Forestry, Sackville, NB, pp. 390–391.
- Woods, C. A., and J. A. Ottenwalder (1983). The montane avifauna of Haiti. In Proceedings of the Jean Delacour/ICFB Symposium on Breeding Birds in Captivity (A. C. Risser Jr. and F. S. Todd, Editors). International Foundation of the Conservation of Birds, Los Angeles, pp. 576–590 & 607–622.
- Woods, C. A., and J. A. Ottenwalder (1986). The birds of Parc National La Visite and Parc National Pic Macaya, Haiti. Report for USAID/Haiti, Contract Number 521-0169-C-OO-3083-00.
- Zelazowski, P., Y. Malhi, C. Huntingford, S. Sitch, and J. B. Fisher (2011). Changes in the potential distribution of humid tropical forests on a warmer planet. Philosophical Transactions, Series A 369:137–160.
Appendix A. Methodological Details
We classified threats and actions using the standardized schemes developed by Salafsky et al. (2008) as modified and implemented by the IUCN Red List Partnership and the Conservation Measures Partnership. We ranked the relative impact of each threat using the IUCN Threat Impact Scoring System. Under this system, the impact of each threat is scored based on whether the threat is ongoing, the proportion of the population affected by the threat, and the expected magnitude of decline caused by the threat. Scores for each component of the impact assessment range from 0-3. We ranked the overall impact of each threat based on the summed value of all components: low-impact threats had summed scores < 4; medium-impact threats had summed scores from 4-6; and high-impact threats had summed scores > 6. High-impact threats are those that are ongoing, affect a large percentage of the population, and are capable of producing steep and rapid declines in numbers within the affected population. Although we categorize threats into high-, medium-, and low-impact, we note that the scoring system recognizes variation in the relative importance of each threat within each category. For example, a medium-impact threat with an overall impact score of 6 is likely to pose a greater risk to Bicknell’s Thrush than a medium-impact threat with a score of 4.
We used professional judgement to classify the feasibility of each action as Extremely Likely to succeed (>90% probability of successful implementation), Likely to succeed (>50% probability of successful implementation), Unlikely to succeed (<50% probability of successful implementation), and Extremely Unlikely to succeed (<10% probability of successful implementation). We classified the effect of an action as either direct and immediate, direct and delayed, or indirect. Actions with direct, immediate effects are expected to directly reduce the negative consequences of the threat within five years; actions with a direct, delayed effect are expected to directly reduce the negative consequences of the threat but with a time lag of >5 years. Actions with an indirect effect on a threat do not directly reduce the negative consequences of the threat, but create conditions that enable mitigation.
Some potentially important threats identified by the IBTCG and its members have a great deal of uncertainty associated with them, primarily in regards to their scope—how much of the population is vulnerable—and severity. This uncertainty precludes ranking the relative degree of risk posed by the threat. All of these threats have additional research as a primary action.